
Ϊ�о����ʲ�������Ũ����ķ�Ӧ��ijѧϰС����̼�ظ�(������̼�ĺϽ�)����������̽�����
[̽��һ]��1������ȥ�������������������̼�ظ֣�������Ũ�����У�10���Ӻ���������ͭ��Һ�У�Ƭ�̺�ȡ���۲죬�������������Ա仯����ԭ���� ��
��2����ȡ̼�ظ�6.0g����15.0mLŨ�����У����ȣ���ַ�Ӧ��õ���ҺX���ռ����������Y��
�ټ�ͬѧ��ΪX�г�Fe3+֮����ܺ���Fe2+����Ҫȷ�����е�Fe2+��Ӧѡ�� ��ѡ����ţ���
A��KSCN��Һ����ˮ B�����ۺ�KSCN��Һ C��Ũ��ˮ D������KMnO4��Һ
����ͬѧȡ560mL(��״��)����Yͨ��������ˮ�У�����SO2+Br2+2H2O=2HBr +H2SO4��Ӧ��Ȼ���������BaCl2��Һ�����ʵ�������ø������4.66g���ɴ���֪����Y��SO2���������Ϊ ��
[̽����]��������ʵ����SO2��������ķ�������ͬѧ��Ϊ����Y�л����ܺ���Q1��Q2�������壬����Q1���壬�ڱ�״���£��ܶ�Ϊ0.0893g��L��1��Ϊ�����������̽��ʵ��װ�ã������й�������ȫ��Ӧ����
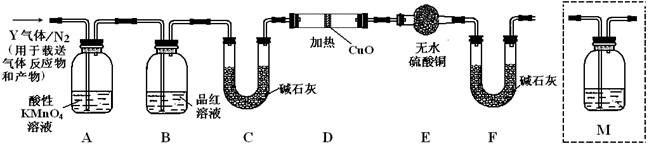
��3��װ��B���Լ���������
��4������Y�����е�Q2������������ɵ� ���û�ѧ����ʽ��ʾ����
��5����֪ϴ��ƿM��ʢװ����ʯ��ˮ��Ϊȷ��Q2�Ĵ��ڣ�������װ��������ϴ��ƿM�� ������ţ���
A��A֮ǰ B��A��B�� C��B��C�� D��C��D��
��6���������Y�к���Q1��Ԥ��ʵ������Ӧ��
��ÿ��2�֣���14�֣�
��1�������ۻ��ˣ� ��2����D�� ��0.80����3������SO2�Ƿ������
��4��C+2H2SO4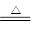 CO2��+2SO2��+2H2O�� ��5��C��
CO2��+2SO2��+2H2O�� ��5��C��
��6��D�еĹ����ɺڱ���E�й����ɰױ�����
����������1�������ڳ����£�����Ũ�����з����ۻ����Ӷ����������γ�һ�����ܵ�����Ĥ�����Բ��ٺ�����ͭ��Һ��Ӧ��
��2�����������Ӿ��л�ԭ�ԣ����Ա����Ը��������Һ�������Ӷ���Һ��ɫ���ݴ˿��Լ��𣬴�ѡD��
�����ᱵ�����ʵ�����0.02mol�������ԭ���غ��֪��SO2�����ʵ���Ҳ��0.02mol��������������ʵ�����0.025mol������SO2�����������0.02��0.025��0.80��
��3���ڱ�״���£��ܶ�Ϊ0.0893g��L��1������������Է���������0.0893��22.4��2.0�����Ը�����������������SO2Ҳ�ܰѼ�ʯ�����գ�����Aװ�õ������dz�ȥSO2����B��Ʒ����Һ�����þ��Ǽ���SO2�Ƿ�����ġ�
��4������Ũ�����ǿ�����ԣ�Ҳ�ܰ�̼��������CO2�����Է�Ӧ�ķ���ʽ��C+2H2SO4 CO2��+2SO2��+2H2O
CO2��+2SO2��+2H2O
��5������CO2Ҳ�ܼ�ʯ�����գ�����Ҫȷ��CO2�Ĵ��ڣ���MӦ�÷���B��C֮�䣬��ѡC��
��6�������������л�ԭ�ԣ��ܰ�����ͭ��ԭ���ɺ�ɫ��ͭ��ˮ��ˮ��ʹ��ˮ����ͭ��������ʵ��������D�еĹ����ɺڱ���E�й����ɰױ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 2SO2��+CO2��+2H2O
2SO2��+CO2��+2H2O 2SO2��+CO2��+2H2O
2SO2��+CO2��+2H2O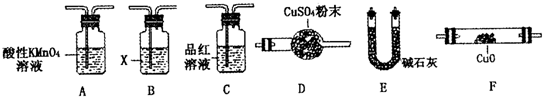
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com