
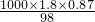 mol/L=15.98 mol?L-1��
mol/L=15.98 mol?L-1�� -2.75mol=1.1mol�����������ʵ���Ϊ19.25mol��
-2.75mol=1.1mol�����������ʵ���Ϊ19.25mol��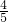 =15.4mol��SO2Ϊ2mol��SO2��������������ʵ�������Ϊ��
=15.4mol��SO2Ϊ2mol��SO2��������������ʵ�������Ϊ��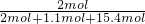 =0.11��
=0.11��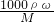 ������������Ϊ0.87��Ũ��������ʵ���Ũ�ȣ�������ˮǰ�����ʵ��������������
������������Ϊ0.87��Ũ��������ʵ���Ũ�ȣ�������ˮǰ�����ʵ��������������


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
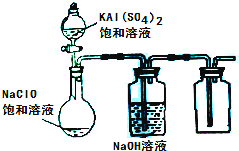 ��2013?����ģ�⣩NaClO��KAl��SO4��2������Ҫ�Ļ�����Ʒ������Ӧ������ֽҵ��
��2013?����ģ�⣩NaClO��KAl��SO4��2������Ҫ�Ļ�����Ʒ������Ӧ������ֽҵ��
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ϻ��г������ζ���������ѧ�ڶ�ģ��ѧ�Ծ��������棩 ���ͣ�������
��������Ҫ�Ļ�����Ʒ���ڻ�����ѧʵ���У�������Ҫ�����á�
��1�����ʵ���Ũ��Ϊ18��4mol/L,��������Ϊ0��98��Ũ��������ˮ����ʱ���������������½���0��87���ܶ�1��8g•cm-3������ʱ����ʧȥ���������� ��������Ϊ0��87����������ʵ���Ũ��Ϊ
��������λС������ͬ����50mL 18��4mol/L��Ũ������Ϊ�����ʱ��������ˮ___________g��
��2����ҵ���Ը����������ᣮ����Ϊԭ����ȡ�����[NH4Al(SO4)2��12H2O]������������Ӧԭ�����£����Ը����������ɷ�������ķ�Ӧ����
Al2O3 + 3H2SO4 �� Al2(SO4)3 + 3H2O����������
Al2(SO4)3 + H2SO4 + 2NH3 �� 2NH4Al(SO4)2 ����������
ij������ͬʱ��ȡ���������������ͨ����������������������ֲ�Ʒ�IJ���������ʹ�Ƶõ�������������������ʵ���֮��Ϊ1:1����Ͷ��ʱ����������������ʵ���֮����
��3�����Ṥҵ�ϴ���ýӴ��������ᣨ��������������������Ϊ20%����Ϊʹ���������ճ�֣���ͨ�����40%�Ŀ����������պ�¯����SO2���������Ϊ_____��
��4��������¯��������������ֱ������Ӵ��ң��������������5%��ͬ��ͬѹ�²ⶨ�����Լ���SO2��ת����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ��ҵ�У���������Ҫ�Ļ�����Ʒ��
��1���������������У����ݻ�ѧƽ��ԭ����ȷ�����������ʩ�� ������ţ���
A����ʯ�������¯֮ǰ�ȷ���
B��V2O5������
C���Ӵ����в�ʹ�úܸߵ��¶�
D������¯����Ҫ�й����Ŀ���
E���Ӵ������ڳ�ѹ�½���
F������������98.3%��Ũ��������SO3
��2����ʵ����537�桢1.01��105Pa�ʹ������������£���ij�ܱ������г���1molSO2��0.5molO2����ʱ���Ϊ100L�����¶Ⱥ��ݻ����������·�Ӧ�ﵽƽ�⣬SO3�������Ϊ 0.91���������¶Ⱥ��ݻ����䣬����1molSO3(q)����ƽ��ʱSO2�������Ϊ ���������¶Ⱥ�ѹǿ���䣬����a molSO2��b molO2����a:b=2:1����Ӧƽ��ʱ�����Ϊ100L��SO3���������Ϊ0.91����a= ��
��3�����Ṥҵ��β���к���������SO2�����ð�ˮ���պ��ټ����ᣬ����SO2ͬʱ�õ���������泥��������Һ�и��������ʵ���Ũ���ɴ�С��˳��Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com