
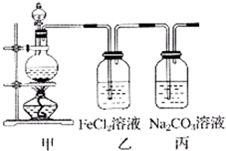
 ��1��ijѧϰС��������ͼװ����ȡ������̽�������ʣ�
��1��ijѧϰС��������ͼװ����ȡ������̽�������ʣ�| ʵ�鲽�� | Ԥ������ͽ��� | |
| �� | ��A�Թ�ȡ��������ʯ��ˮ���ã���B�Թ�ȡ������Ʒ������B�Թ�______ | ��������ų��ҳ���ʯ��ˮδ�����ǣ������һ������______ |
| �� | ______ | ______ |
 MnCl2+Cl2��+2H2O��
MnCl2+Cl2��+2H2O�� MnCl2+Cl2��+2H2O��
MnCl2+Cl2��+2H2O��| ʵ�鲽�� | Ԥ������ͽ��� | |
| �� | ����B�Թܼ���1mol/L��ϡ�����������1mol/L�����ᣬ���ϴ����ܵĵ������������ܵ���һ�˲���A�Թ��� | ��������ð�����ҳ���ʯ��ˮ����������һ��������������������� |
| �� | �ý�ͷ�ι�������������B�Թ��е��뼸��Ʒ����Һ���� | ��Ʒ����Һ����ɫ����������������Ʒ����Һ��ɫ������������� |


 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
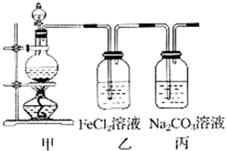 ��2013?����һģ����1��ijѧϰС��������ͼװ����ȡ������̽�������ʣ�
��2013?����һģ����1��ijѧϰС��������ͼװ����ȡ������̽�������ʣ�
| ||
| ||
| ʵ�鲽�� | Ԥ������ͽ��� | |
| �� | ��A�Թ�ȡ��������ʯ��ˮ���ã���B�Թ�ȡ������Ʒ������B�Թ� ��������1mol/L�����ᣬ���ϴ����ܵĵ������������ܵ���һ�˲���A�Թ��� ��������1mol/L�����ᣬ���ϴ����ܵĵ������������ܵ���һ�˲���A�Թ��� |
��������ų��ҳ���ʯ��ˮδ�����ǣ������һ������ ��������ð�����ҳ���ʯ��ˮ����������һ��������������������� ��������ð�����ҳ���ʯ��ˮ����������һ��������������������� |
| �� | �ý�ͷ�ι�������������B�Թ��е��뼸��Ʒ����Һ���� �ý�ͷ�ι�������������B�Թ��е��뼸��Ʒ����Һ���� |
��Ʒ����Һ����ɫ����������������Ʒ����Һ��ɫ������������� ��Ʒ����Һ����ɫ����������������Ʒ����Һ��ɫ������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�����и�����һ�Σ�3�£�ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�������
��1��ijѧϰС��������ͼװ����ȡ������̽�������ʡ�
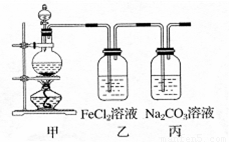
�ټ�װ���з�Ӧ�Ļ�ѧ����ʽ�� ��
��֤����װ����FeCl2��Һ��Cl2�����˷�Ӧ��ʵ�鷽���ǣ�ֻע���Լ������� ��
�۱�װ����ͨ������Cl2�����Ƶ�ij�������г��õ�Ư�ס����������ʡ���֪̼�������ǿ�ڴ����ᣬ����з�Ӧ�Ļ�ѧ����ʽ�� ��
��2����һƿ���ڷ��õ�Ư�ۣ������������������Լ�����ɸ�Ư�۳ɷݵ�̽����
�Թܡ���ͷ�ιܡ������ܵĵ�����������ˮ��1mol��L-1���ᡢƷ����Һ�����Ƴ���ʯ��ˮ��
��������衿����һ����Ư��δ���ʣ���CaCl2��Ca��ClO��2��
���������Ư��ȫ�����ʣ��� ��
����������Ư�۲��ֱ��ʣ���CaCl2��Ca��ClO��2��CaCO3 ��
������ʵ�顿�ڴ��������±������ؼ���Ca2+��Cl-����
|
ʵ�鲽�� |
Ԥ������ͽ��� |
|
|
�� |
��A�Թ�ȡ��������ʯ��ˮ���ã���B�Թ�ȡ������Ʒ������B�Թ�
|
��������ų��ҳ���ʯ��ˮδ�����ǣ������һ������
|
|
�� |
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����Զģ�� ���ͣ��ʴ���

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com