
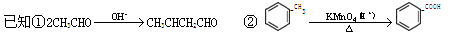
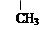 3��������Ӧ����ȥ��Ӧ��©����Ӧ����1�֣� 4��CH3-CH-COOH
3��������Ӧ����ȥ��Ӧ��©����Ӧ����1�֣� 4��CH3-CH-COOH |



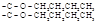 +2H2O
+2H2O

 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

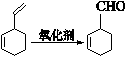
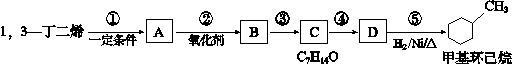
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
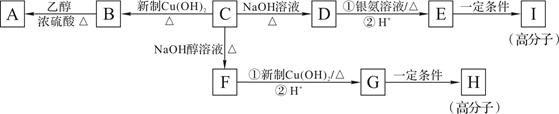
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
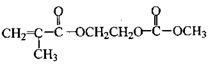
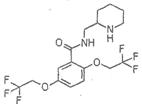
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
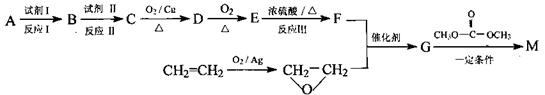
 �������������ᣨ
�������������ᣨ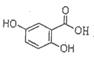 ��Ϊԭ�Ϻϳɣ��ϳɵķ�������ͼ��
��Ϊԭ�Ϻϳɣ��ϳɵķ�������ͼ��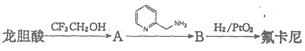
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

| ���� | �е� | �ܽ��� | ��Ҫ��ѧ���� |
| ����� | 152.4�� | ������ˮ�������ھƾ��� | �ɱ�ǿ���������� |
| ���� | 181.8�� | ������ˮ����������ˮ�;ƾ����л��ܼ��� | �ױ����� |
| ��ͪ | 56.2�� | ������ˮ���л��ܼ� | ���ױ����� |
| ������Ŀ | ʵ�鷽�� |
| �����ͪ��һ�������л����ʵķ����� | A������KMnO4��Һ������ B���Ҵ����ܽ� C��NaOH��Һ����̪�������� |
| �����ͪ��һ�����б��ӵķ����ǣ� | ȡ��������Һ�����Թ��У��μ�1��2��FeCl3��Һ��Ԥ�ڵ�ʵ������ͽ����� �� |
 + 28KMnO4 + 42H2SO4
+ 28KMnO4 + 42H2SO4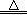 28MnSO4 + 14K2SO4 + 30CO2��+ 57H2O
28MnSO4 + 14K2SO4 + 30CO2��+ 57H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
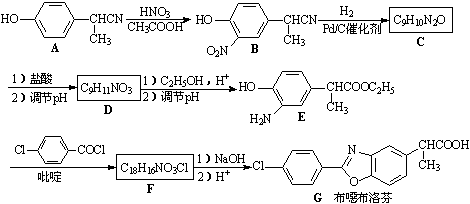
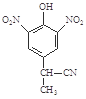 b��
b��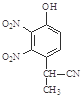 c��
c��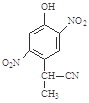 d��
d��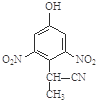
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
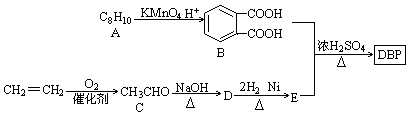
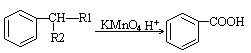
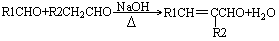
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com