
����ȡ��Ӧ��ʱ��ȡ50mL0.50mol��L-1�����ᣬ��Ӧ������Լ���________(�����)��
A.50mL0.50mol��L-1NaOH��Һ
B.50mL0.55mol��L-1NaOH��Һ
C.1.
����ʵ������У���ͬѧ��Ҫ�ⶨ����¼��ʵ��������__________(�����)��
A.�����Ũ��
B.������¶�
C.����������Һ��Ũ��
D.����������Һ���¶�
E.ˮ�ı�����
F.��Ӧ������Һ����ֹ�¶�
������50mL0.5mol��L-1������Һ������������ⶨ�к��ȣ���������_________(�ƫ��ƫС�����䡱)��
(2)����ͭ����(CuSO4��xH2O)�ᾧˮ�����IJⶨʵ�顣
�ٳ���һ����������ͭ��������ѽᾧˮʱ�õ���������Ҫ�У������������żܡ�����������ǯ�������Ǻ�_________��
��ʵ����Ҫ���������ڸ���������ȴ������ҪĿ����______________________________________________________��
��������������Ϊm������������ͭ���������Ϊm1�����Ⱥ���������ˮ����ͭ������Ϊm2������CuSO4��xH2O�У�x=_________(д����ʽ)��
�����ʵ���м����¶ȹ��ߣ�������ɫ��ڣ���ⶨ�����_________(����䡱��ƫ�ߡ���ƫ�͡�)��
(1)��B ��B��D��F ��ƫС
(2)�پƾ��� �ڷ�ֹ��ˮ����ͭ�ڿ���������ˮ����ʹ��������� ��![]() ��ƫ��
��ƫ��
�����������⿼�����к��Ȳⶨʵ�飬Ϊʹ������ȫ��Ӧ��Ӧ���Թ�����NaOH��Һ����ʵ���ص���Dz����¶ȣ����������¶���T1����֤NaOH��Һ�¶���T1��ȣ������Ӧ�ﵽ����¶�ʱ��T2����������Ҫ�Ƿ�Ӧ�ų�����������ʧ������ô��ᣬ�ڷ�Ӧ�д���Ҫ���룬�����������������������ݻ�ƫС��


 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д� ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
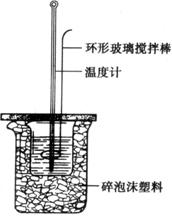
��8�֣�ij��ѧ��ȤС��Ҫ����к��ȵIJⶨ��
��1��ʵ�����ϱ����ձ�����С�����ձ�������ĭ���ϡ���ĭ���ϰ塢��ͷ�ιܡ����β�������0.5mol�� L-1 ���ᡢ0.55mol�� L-1NaOH��Һ����ȱ�ٵ�ʵ�鲣����Ʒ�� �� ��
��2�����Ǽ�¼��ʵ���������£�
| ʵ �� �� Ʒ | �� Һ �� �� | �к��ȡ�H | |||
| t1 | t2 | ||||
| �� | 50mL0.55mol.L-1NaOH | 50mL.0.5mol.L-1HCl | 20�� | 23.3�� |
|
| �� | 50mL0.55mol.L-1NaOH | 50mL.0.5mol.L-1HCl | 20�� | 23.5�� |
��֪��Q=Cm(t2 -t1)����Ӧ����Һ�ı�����CΪ4.18KJ����-1�� Kg-1�������ʵ��ܶȾ�Ϊ1g��cm-3��
��������ϱ�����H=
��3��ij�о�С�齫V1 mL 1.0 mol/L HCl��Һ��V2 mLδ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ��������¼��Һ�¶ȣ�ʵ��������ͼ��ʾ(ʵ����ʼ�ձ���V1��V2��50 mL)���ش��������⣺
�о�С������ʵ��ʱ�����¶� (����ڡ��������ڡ����ڡ�)22 �棬�˷�Ӧ����NaOH��Һ��Ũ��ӦΪ mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ��ȤС��Ҫ����к��ȵIJⶨ��
��1��ʵ�����ϱ����ձ�����С�����ձ�������ĭ���ϡ���ĭ���ϰ塢��ͷ�ιܡ����β�������0.5mol�� L-1 ���ᡢ0.55mol�� L-1NaOH��Һ����ȱ�ٵ�ʵ�鲣����Ʒ�� �� ��
��2��ʵ�����ܷ��û���ͭ˿��������滷�β����������
����ܡ�������ԭ���� ��
��3�����Ǽ�¼��ʵ���������£�
| ʵ �� �� Ʒ | �� Һ �� �� | �к��� ��H | |||
| t1 | t2 | ||||
| �� | 50mL0.55mol.L-1NaOH | 50mL.0.5mol.L-1HCl | 20�� | 23.3�� |
|
| �� | 50mL0.55mol.L-1NaOH | 50mL.0.5mol.L-1HCl | 20�� | 23.5�� |
��֪��Q=Cm(t2 -t1)����Ӧ����Һ�ı�����CΪ4.18KJ����-1�� Kg-1�������ʵ��ܶȾ�Ϊ1g��cm-3��
����ʵ����д��NaOH��Һ��HCl��Һ��Ӧ���Ȼ�ѧ����ʽ��
��4������KOH����NaOH���Բⶨ��� ����С����ޡ���Ӱ�졣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013�꺣�������λ���ѧ�߶��ϸ��н�ѧ�����������ѧ�Ծ����������� ���ͣ������
��10�֣�ij��ѧ��ȤС��Ҫ����к��ȵIJⶨ��
��1��ʵ�����ϱ����ձ�����С�����ձ�������ĭ���ϡ���ĭ���ϰ塢��ͷ�ιܡ����β����������0.5mol�� L-1���ᡢ0.55mol�� L-1NaOH��Һ��
��ȱ�ٵ�ʵ�鲣����Ʒ�� �� ��
��2��ʵ�����ܷ��û���ͭ˿��������滷�β����������
����ܡ�������ԭ���� ��
��3�����Ǽ�¼��ʵ���������£�
| ʵ �� �� Ʒ | �� Һ �� �� | �к��� ��H | |||
| t1 | t2 | ||||
| �� | 50mL0.55mol.L-1NaOH | 50mL.0.5mol.L-1HCl | 20�� | 23.3�� | |
| �� | 50mL0.55mol.L-1NaOH | 50mL.0.5mol.L-1HCl | 20�� | 23.5�� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��8�֣� ij��ѧ��ȤС��Ҫ����к��ȵIJⶨ��
��1��ʵ�����ϱ����ձ�����С�����ձ�������ĭ���ϡ���ĭ���ϰ塢��ͷ�ιܡ����β�������0.5mol�� L-1 ���ᡢ0.55mol�� L-1NaOH��Һ����ȱ�ٵ�ʵ�鲣����Ʒ�� �� ��
��2�����Ǽ�¼��ʵ���������£�
|
ʵ �� �� Ʒ |
�� Һ �� �� |
�����H |
|||
|
t1 |
t2 |
||||
|
�� |
50mL0.55mol.L-1NaOH |
50mL.0.5mol.L-1HCl |
20�� |
23.3�� |
|
|
�� |
50mL0.55mol.L-1NaOH |
50mL.0.5mol.L-1HCl |
20�� |
23.5�� |
��֪��Q=Cm(t2 -t1)����Ӧ����Һ�ı�����CΪ4.18KJ����-1�� Kg-1�������ʵ��ܶȾ�Ϊ1g��cm-3��
��������ϱ�����H=
��3��ij�о�С�齫V1 mL 1.0 mol/L HCl��Һ��V2 mLδ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ��������¼��Һ�¶ȣ�ʵ��������ͼ��ʾ(ʵ����ʼ�ձ���V1��V2��50 mL)���ش��������⣺
�о�С������ʵ��ʱ�����¶� (����ڡ��������ڡ����ڡ�)22 �棬�˷�Ӧ����NaOH��Һ��Ũ��ӦΪ mol/L��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㽭ʡ2009-2010ѧ��ڶ�ѧ�ڸ߶�3���¿���ѧ�Ծ� ���ͣ�ʵ����
ij��ѧ��ȤС��Ҫ����к��ȵIJⶨ��
��1��ʵ�����ϱ����ձ�����С�����ձ�������ĭ���ϡ���ĭ���ϰ塢��ͷ�ιܡ����β�������0.5mol�� L-1 ���ᡢ0.55mol�� L-1NaOH��Һ����ȱ�ٵ�ʵ�鲣����Ʒ�� �� ��
��2��ʵ�����ܷ��û���ͭ˿��������滷�β����������
����ܡ�������ԭ���� ��
��3�����Ǽ�¼��ʵ���������£�
|
ʵ �� �� Ʒ |
�� Һ �� �� |
��� ��H |
|||
|
t1 |
t2 |
||||
|
�� |
50mL0.55mol.L-1NaOH |
50mL.0.5mol.L-1HCl |
20�� |
23.3�� |
|
|
�� |
50mL0.55mol.L-1NaOH |
50mL.0.5mol.L-1HCl |
20�� |
23.5�� |
��֪��Q=Cm(t2 -t1)����Ӧ����Һ�ı�����CΪ4.18KJ����-1�� Kg-1�������ʵ��ܶȾ�Ϊ1g��cm-3��
����ʵ����д��NaOH��Һ��HCl��Һ��Ӧ���Ȼ�ѧ����ʽ��
��4������KOH����NaOH���Բⶨ��� ����С����ޡ���Ӱ�졣
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com