
”¾ĢāÄæ”æŅŃÖŖĄ¶ĶæóµÄÖ÷ŅŖ³É·ÖŹĒ2CuCO3”¤Cu(OH)2£¬ŹÜČČŅ×·Ö½ā”£ĀĮĶĮæóµÄÖ÷ŅŖ³É·ÖŹĒAl2O3”¢Fe2O3”¢SiO2”£ŅŃÖŖ:NaAlO2+CO2+H2O = Al(OH)3”ż+NaHCO3£¬øł¾ŻĻĀĮŠæņĶ¼×Ŗ»Æ»Ų“šĪŹĢā£ŗ
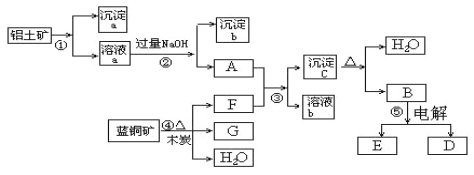
£Ø1£©Š“³ö¢ŚµÄĄė×Ó·½³ĢŹ½£ŗ_________________________”¢_____________________________”£
£Ø2£©³Įµķa”¢c»Æѧ³É·Ö·Ö±šŹĒ£ŗ ___________________”¢_________________________
£Ø3£©ĒėŠ“³ö¼ģŃé³ĮµķbÖŠĖłŗ¬ÓŠŃōĄė×ӵďµŃé·½·Ø_____________________________________________________________”£
£Ø4£©Ļ“µÓ³ĮµķcµÄŹµŃé²Ł×÷·½·ØŹĒ_______________________________________________________£»¼ÓČČ³ĮµķcÓ¦·ÅŌŚ___________£ØČŻĘ÷£©ÖŠ½ųŠŠ”£
£Ø5£©¾¹ż¢Ü”¢¢Ż²½·“Ó¦µĆµ½ĶŗĶ½šŹōĀĮ£¬Š“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ________________________________________________”¢____________________________________”£
”¾“š°ø”æAl3+ + 4OH”Ŗ = AlO2”Ŗ+ 2H2OFe3+ + 3OH”Ŗ= Fe(OH)3”żSiO2Al(OH)3Č”³ĮµķÉŁĮæÓŚŅ»Ö§½ą¾»µÄŹŌ¹ÜÖŠ£¬¼ÓČėÉŁĮæŃĪĖį£¬Č»ŗóŌŁĶłŹŌ¹ÜÖŠ¼ÓČė¼øµĪµÄKSCNČÜŅŗ£¬·¢ĻÖŹŌ¹ÜÄŚ³ŹĻÖŗģÉ«ĻņĀ©¶·ÄŚµÄ³ĮµķÉĻ¼ÓČėŅ»¶ØĮæµÄÕōĮóĖ®£¬Ć»¹ż³Įµķ£¬“żĖ®×ŌČ»Į÷ĻĀ£¬ÖŲø“Źż2”Ŗ3“ĪŪįŪö2 (2CuCO3”¤Cu(OH)2)+3C![]() 6Cu + 7CO2”ü+ 2H2O2Al2O3
6Cu + 7CO2ӟ+ 2H2O2Al2O3![]() 4Al+3O2ӟ
4Al+3O2ӟ
”¾½āĪö”æ
ĀĮĶĮæóµÄÖ÷ŅŖ³É·ÖŹĒAl2O3”¢Fe2O3”¢SiO2£¬ČÜŅŗaÄÜÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦£¬Ó¦ŗ¬ÓŠĀĮĄė×Ó”¢ĢśĄė×Ó£¬ŌņĀĮĶĮæó¼ÓČėŃĪĖį£¬Čܽā¹żĀĖµĆµ½³ĮµķaĪŖSiO2£¬ČÜŅŗaĪŖĀČ»ÆĀĮ”¢ĀČ»ÆĢśČÜŅŗ£¬¼ÓČė¹żĮæĒāŃõ»ÆÄĘČÜŅŗ£¬¹żĀĖµĆµ½³ĮµķbĪŖFe£ØOH£©3£¬AĪŖNaAlO2ČÜŅŗ£¬Ą¶ĶæóµÄÖ÷ŅŖ³É·ÖŹĒ2CuCO3Cu£ØOH£©2£¬ŹÜČČŅ×·Ö½ā¼ÓČėľĢ滹ŌÉś³É²śĪļĪŖFĪŖCO2£¬GĪŖCu£¬F+A·“Ӧɜ³É³ĮµķcĪŖAl£ØOH£©3£¬ČÜŅŗbĪŖĢ¼ĖįĒāÄĘČÜŅŗ£¬³ĮµķcŹÜČČ·Ö½āµĆµ½BĪŖAl2O3ŗĶĖ®£¬Ńõ»ÆĀĮµē½āµĆµ½EŗĶDĪŖŃõĘųŗĶĀĮ£¬¾Ż“Ė·ÖĪöæÉµĆ½įĀŪ”£
£Ø1£©·“Ó¦¢ŚŹĒĒāŃõ»ÆÄĘČÜŅŗŗĶĀČ»ÆĀĮČÜŅŗ·“Ӧɜ³ÉĘ«ĀĮĖįÄĘ£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗAl3++4OH-=AlO2-+2H2O£¬ĒāŃõ»ÆÄĘČÜŅŗŗĶĀČ»ÆĢś·“Ӧɜ³ÉĒāŃõ»ÆĢś³Įµķ£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗFe3++3OH-=Fe£ØOH£©3”ż£¬¹Ź“š°øĪŖ£ŗAl3++4OH-=AlO2-+2H2O”¢Fe3++3OH-=Fe£ØOH£©3”ż£»£Ø2£©ÓÉÉĻŹö·ÖĪöæÉÖŖaĪŖ¶žŃõ»Æ¹č»ÆѧŹ½ĪŖSiO2£¬cĪŖĒāŃõ»ÆĀĮ»ÆѧŹ½ĪŖAl£ØOH£©3£¬¹Ź“š°øĪŖ£ŗSiO2£»Al£ØOH£©3£»£Ø3£©Ļ“µÓ³ĮµķcµÄŹµŃé²Ł×÷·½·ØŹĒ£ŗŌŚĀ©¶·ÄŚµÄ³ĮµķÉĻ¼ÓČėŅ»¶ØĮæµÄÕōĮóĖ®£¬Ć»¹ż³Įµķ£¬“żĖ®×ŌČ»Į÷ĻĀ£¬ÖŲø“Źż“Ī£¬¹ĢĢåĪļÖŹ×ĘÉÕŅ»°ćŌŚŪįŪöÖŠ½ųŠŠ£¬¹Ź“š°øĪŖ£ŗŌŚĀ©¶·ÄŚµÄ³ĮµķÉĻ¼ÓČėŅ»¶ØĮæµÄÕōĮóĖ®£¬Ć»¹ż³Įµķ£¬“żĖ®×ŌČ»Į÷ĻĀ£¬ÖŲø“Źż“Ī£»ŪįŪö£»£Ø4£©Ą¶ĶæóÓėľĢæ·“Ó¦µÄ·½³ĢŹ½ĪŖ£ŗ2 (2CuCO3”¤Cu(OH)2)+3C![]() 6Cu + 7CO2”ü+ 2H2O£¬µē½āČŪČŚŃõ»ÆĀĮµĆµ½ĀĮŗĶŃõĘų·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ2Al2O3
6Cu + 7CO2”ü+ 2H2O£¬µē½āČŪČŚŃõ»ÆĀĮµĆµ½ĀĮŗĶŃõĘų·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ2Al2O3![]() 4Al+3O2”ü£¬¹Ź“š°øĪŖ£ŗ2 (2CuCO3”¤Cu(OH)2)+3C
4Al+3O2”ü£¬¹Ź“š°øĪŖ£ŗ2 (2CuCO3”¤Cu(OH)2)+3C![]() 6Cu + 7CO2”ü+ 2H2O”¢2Al2O3
6Cu + 7CO2ӟ+ 2H2OӢ2Al2O3![]() 4Al+3O2ӟӣ
4Al+3O2ӟӣ


 ·¢É¢Ė¼Ī¬ŠĀæĪĢĆĻµĮŠ“š°ø
·¢É¢Ė¼Ī¬ŠĀæĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”ææÉÄę·“Ó¦£ŗaA£Øg£©+bB£Øg£©![]() cC£Øg£©+dD£Øg£©£»øł¾ŻĶ¼»Ų“š£ŗ
cC£Øg£©+dD£Øg£©£»øł¾ŻĶ¼»Ų“š£ŗ

£Ø1£©Ń¹Ēæp1±Čp2 £ØĢī”°“ó”±»ņ”°Š””±£©
£Ø2£©£Øa+b£©±Č£Øc+d£© £ØĢī”°“ó”±»ņ”°Š””±£©
£Ø3£©ĪĀ¶Čt1±Čt2”ę £ØĢī”°øß”±»ņ”°µĶ”±£©
£Ø4£©Õż·“Ó¦ĪŖ ČČ·“Ó¦£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ»ĘĶæó£ØCuFeS2£©ŹĒĮ¶ĶµÄ×īÖ÷ŅŖæóĪļ”£»š·ØŅ±Į¶»ĘĶæóµÄ¹ż³ĢÖŠ£¬ĘäÖŠŅ»²½·“Ó¦ŹĒ£»2Cu2O+ Cu2S![]() 6Cu+SO2”£»Ų“šĻĀĮŠĪŹĢā”£
6Cu+SO2”£»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©Cu+µÄ¼Ūµē×Ó¹ģµĄ±ķŹ¾Ź½ĪŖ__________________£»Cu2OÓėCu2S±Č½Ļ£¬ČŪµć½ĻøߵďĒ_______£¬ŌŅņĪŖ_____________________________________”£
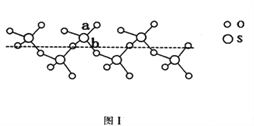
£Ø2£©SO2ÓėSO3µÄ¼ü½ĒĻą±Č£¬¼ü½Ēøü“óµÄŹĒ____________”£½«“æŅŗĢ¬SO3ĄäČ“µ½289.8KŹ±Äż¹ĢµĆµ½Ņ»ÖÖĀŻŠż×“µ„Į“½į¹¹µÄ¹ĢĢ壬Ęä½į¹¹ČēĻĀĶ¼1ĖłŹ¾”£“Ė¹ĢĢ¬SO3ÖŠSŌ×ÓµÄŌӻƹģµĄĄąŠĶŹĒ_______£»øĆ½į¹¹ÖŠS”ŖO¼ü³¤ÓŠĮ½Ąą£¬Ņ»Ąą¼ü³¤Ō¼140pm£¬ĮķŅ»Ąą¼ü³¤Ō¼ĪŖ160pm£¬½Ļ¶ĢµÄ¼üĪŖ_________£ØĢīĶ¼ÖŠ×ÖÄø£©”£
£Ø3£©Ąė×Ó»ÆŗĻĪļCaC2µÄŅ»ÖÖ¾§Ģå½į¹¹ČēĻĀĶ¼2ĖłŹ¾”£Š“³öøĆĪļÖŹµÄµē×ÓŹ½_____”£“ÓøĘĄė×Óæ“£¬ŹōÓŚ____________¶Ń»ż£»Ņ»øö¾§°ūŗ¬ÓŠµÄ¦Š¼üĘ½¾łÓŠ______øö”£
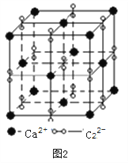

£Ø4£©°ĀŹĻĢåŹĒĢ¼ČܽāŌŚ¦Ć”ŖFeÖŠŠĪ³ÉµÄŅ»ÖÖ¼äĻ¶¹ĢČÜĢ壬ĪŽ“ÅŠŌ£¬Ę侧°ūČēÉĻĶ¼3ĖłŹ¾£¬ŌņøĆĪļÖŹµÄ»ÆѧŹ½ĪŖ________£¬Čō¾§ĢåĆܶČĪŖdg/cm3£¬Ōņ¾§°ūÖŠ×ī½üµÄĮ½øöĢ¼Ō×ӵľąĄėĪŖ____________________ pm”££Ø°¢·ü¼ÓµĀĀŽ³£ŹżµÄÖµÓĆNA±ķŹ¾£¬Š“³ö¼ĘĖćŹ½¼“æÉ£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĆ÷·Æ[ KAl(SO4)2”¤12H2O ]ŌŚŌģÖ½”¢¾»Ė®µČ·½ĆęÓ¦ÓĆ¹ć·ŗ”£ŅŌ“¦Ąķ¹żµÄ·Ļ¾ÉŅץ¹ŽĖ銼ĪŖŌĮĻ(Ö÷ŅŖ³É·ÖĪŖAl£¬ŗ¬ÓŠÉŁĮæµÄFe”¢MgŌÓÖŹ)ÖʱøĆ÷·ÆµÄ¹ż³ĢČēĻĀĶ¼ĖłŹ¾”£

»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ŌĮĻČܽā¹ż³ĢÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ_________________________”£
(2)Al(OH)3 ÓėĻ”ĮņĖį·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ__________________________”£
(3)ČÜŅŗAÖŠĶØČė¹żĮæCO2£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ_________________”£
(4)ĪŖÖ¤Ć÷¹ĢĢåBÖŠŗ¬ÓŠĢś£¬Ä³Ķ¬Ń§×öČēĻĀŹµŃé£ŗȔɣĮæ¹ĢĢåB£¬¼ÓČėĻ”ĮņĖįŹ¹ĘäČܽā£¬¹Ū²ģµ½ÓŠĪŽÉ«ĘųĢåÉś³É”£ĻņČÜŅŗÖŠ¼ÓČė___________£¬ČÜŅŗĮ¢¼“±äŗģ£¬Ö¤Ć÷¹ĢĢåBÖŠŗ¬ÓŠĢś”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻÖÓŠŅ»¶ØĮæŗ¬ÓŠNa2OŌÓÖŹµÄNa2O2ŹŌŃł£¬ÓĆČēĶ¼µÄŹµŃé×°ÖĆ²ā¶ØNa2O2ŹŌŃłµÄ“æ¶Č”£
»Ų“šĻĀĮŠĪŹĢā£ŗ

(1)×°ÖĆAÖŠŹ¢·ÅĻ”ŃĪĖįµÄŅĒĘ÷Ćū³ĘĪŖ__________________”£
(2)×°ÖĆBµÄ×÷ÓĆŹĒ______________________________________”£
(3)×°ÖĆCµÄ×÷ÓĆŹĒ____________________________________________”£
(4)×°ÖĆDÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ_____________”¢_______________”£
(5)×°ÖĆEÖŠ¼īŹÆ»ŅµÄ×÷ÓĆŹĒ______________________________________”£
(6)ČōæŖŹ¼Ź±³ĘµĆѳʷµÄÖŹĮæĪŖ4.52 g£¬·“Ó¦½įŹųŗó³ĘµĆ¹ĢĢåµÄÖŹĮæĪŖ6.36g£¬ŌņNa2O2ŹŌŃłµÄ“æ¶ČĪŖ____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ·ÖĪöČēĶ¼ĖłŹ¾µÄĖÄøöŌµē³Ų×°ÖĆ£¬ĘäÖŠ½įĀŪÕżČ·µÄŹĒ(””””)

A. ¢Ł¢ŚÖŠMg×÷ĪŖøŗ¼«£¬¢Ū¢ÜÖŠFe×÷ĪŖøŗ¼«
B. ¢ŚÖŠMg×÷ĪŖÕż¼«£¬µē¼«·“Ó¦Ź½ĪŖ6H2O£«6e£===6OH££«3H2”ü
C. ¢ŪÖŠFe×÷ĪŖøŗ¼«£¬µē¼«·“Ó¦Ź½ĪŖFe£2e£===Fe2£«
D. ¢ÜÖŠCu×÷ĪŖÕż¼«£¬µē¼«·“Ó¦Ź½ĪŖ2H£«£«2e£===H2”ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”潚øÕŹÆŗĶŹÆÄ«¾łĪŖĢ¼µÄĶ¬ĖŲŅģŠĪĢ壬ĖüĆĒČ¼ÉÕŃõĘų²»×揱ɜ³ÉŅ»Ńõ»ÆĢ¼£¬³ä·ÖČ¼ÉÕÉś³É¶žŃõ»ÆĢ¼£¬·“Ó¦ÖŠ·Å³öµÄČČĮæČēĶ¼ĖłŹ¾£®
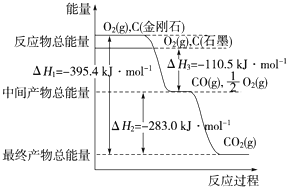
£Ø1£©µČĮ潚øÕŹÆŗĶŹÆÄ«ĶźČ«Č¼ÉÕ___£ØĢī”°½šøÕŹÆ”±ŗĶ”°ŹÆÄ«”±£©·Å³öČČĮæøü¶ą£¬Š“³öŹÆÄ«ĶźČ«Č¼ÉÕµÄČČ»Æѧ·½³ĢŹ½___”£
£Ø2£©ŌŚĶس£×“æöĻĀ£¬½šøÕŹÆŗĶŹÆÄ«___£ØĢī”°½šøÕŹÆ”±ŗĶ”°ŹÆÄ«”±£©øüĪČ¶Ø£¬Š“³öŹÆÄ«×Ŗ»ÆĪŖ½šøÕŹÆµÄČČ»Æѧ·½³ĢŹ½£ŗ_______________”£
£Ø3£©12gŹÆÄ«ŌŚŅ»¶ØĮææÕĘųÖŠČ¼ÉÕ£¬Éś³ÉĘųĢå36g£¬øĆ¹ż³Ģ·Å³öµÄČČĮæĪŖ___”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŌŚĻĀĮŠø÷±ä»ÆÖŠ£¬·“Ó¦¢ŁĪŖ³£ĪĀĻĀµÄ·“Ó¦£¬A”¢C”¢D¾łŗ¬ĀČŌŖĖŲ£¬ĒŅAÖŠĀČŌŖĖŲµÄ»ÆŗĻ¼Ū½éÓŚCÓėDÖ®¼ä£¬E³£ĪĀĻĀĪŖĪŽÉ«ĪŽĪ¶µÄŅŗĢ壬FĪŖµ»ĘÉ«·ŪÄ©£¬GĪŖ³£¼ūµÄĪŽÉ«ĘųĢ唣
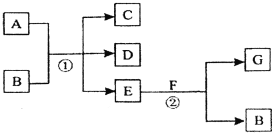
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)A”¢GµÄ»ÆѧŹ½·Ö±šĪŖ________________”¢ ________________”£
(2)FŗĶE·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ____________________________”£
(3)Š“³ö·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½____________________________________”£
(4)ŌŚ·“Ó¦¢ŚÖŠ£¬ĆæÉś³É2.24 LĘųĢåG(±ź×¼×“æö)Ź±£¬ĻūŗÄF ___________g”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ°Ńŗ¬ĮņĖįļ§ŗĶĻõĖįļ§µÄ»ģŗĻŅŗa L·Ö³ÉĮ½µČ·Ż£®Ņ»·Ż¼ÓČėŗ¬b mol NaOHµÄČÜŅŗ²¢¼ÓČČ£¬Ē”ŗĆ°ŃNH3Č«²æøĻ³ö£»ĮķŅ»·ŻŠčĻūŗÄc mol BaCl2²ÅÄÜŹ¹SO42£ĶźČ«³Įµķ£¬ŌņŌČÜŅŗÖŠNO3£µÄĪļÖŹµÄĮæÅضČĪŖ£Ø £©
A£®![]() B£®
B£® ![]() C£®
C£® ![]() D£®
D£® ![]()
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com