
ʵ���ҴӺ����Һ(��H2O�⣬����CCl4��I2��I����)�л��յ⣬��ʵ��������£�

(1)���Һ�м����Թ�����Na2SO3��Һ������Һ�е�I2��ԭΪI���������ӷ���ʽΪ__________________���ò�����I2��ԭΪI����Ŀ����______________________��
(2)����X������Ϊ________��
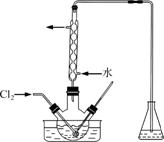
(3)����ʱ����������ƿ�н���I����ˮ��Һ���������pHԼΪ2������ͨ��Cl2����40 �����ҷ�Ӧ(ʵ��װ����ͼ��ʾ)��
ʵ������ڽϵ��¶��½��е�ԭ����______________����ƿ��ʢ�ŵ���ҺΪ________��
(4)��֪��5SO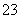 ��2IO
��2IO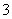 ��2H��===I2��5SO
��2H��===I2��5SO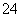 ��H2O
��H2O
ij�����ˮ(pHԼΪ8)��һ������I2�����ܴ���I����IO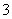 �е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I����IO
�е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I����IO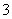 ��ʵ�鷽����ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���еⵥ�ʴ��ڣ�________________________________________________________________________
��ʵ�鷽����ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���еⵥ�ʴ��ڣ�________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
ʵ���пɹ�ѡ����Լ���ϡ���ᡢ������Һ��FeCl3��Һ��Na2SO3��Һ��
(1)SO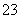 ��I2��H2O===2I����SO
��I2��H2O===2I����SO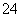 ��2H����ʹCCl4�еĵ����ˮ��
��2H����ʹCCl4�еĵ����ˮ��
(2)��Һ
(3)ʹ��������Һ���нϴ���ܽ��(���ֹI2�������ֹI2��һ��������)��NaOH��Һ
(4)��ˮ��ȡ������Һ������1��2 mL������Һ���������ữ���μ�FeCl3��Һ������Һ������˵����ˮ�к���I��������Һ��������˵����ˮ�в�����I��������ˮ��ȡ������Һ������1��2 mL������Һ���������ữ���μ�Na2SO3��Һ������Һ������˵����ˮ�к���IO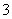 ������Һ��������˵����ˮ�в�����IO
������Һ��������˵����ˮ�в�����IO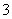
[����] (1)SO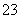 ��I2����ΪSO
��I2����ΪSO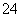 ��I2����ԭΪI������ϵ���غ��ԭ���غ�ɵ�SO
��I2����ԭΪI������ϵ���غ��ԭ���غ�ɵ�SO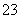 ��I2��H2O===2I����SO
��I2��H2O===2I����SO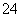 ��2H������ΪI2������ˮ�����⻯��������ˮ���ʽ�I2��ԭΪI����Ŀ����ʹ��Ԫ�ؽ���ˮ�㡣(2)�����л��ܼ���ˮ��Һ�Ļ������Ҫ��Һ��(3)�¶�Խ�ߣ�Cl2�ܽ��ԽС���������¶����ߣ�Cl2���I2��һ������ΪIO
��2H������ΪI2������ˮ�����⻯��������ˮ���ʽ�I2��ԭΪI����Ŀ����ʹ��Ԫ�ؽ���ˮ�㡣(2)�����л��ܼ���ˮ��Һ�Ļ������Ҫ��Һ��(3)�¶�Խ�ߣ�Cl2�ܽ��ԽС���������¶����ߣ�Cl2���I2��һ������ΪIO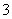 ����������I����Ч��ƫ�ͣ����⣬I2Ҳ����������
����������I����Ч��ƫ�ͣ����⣬I2Ҳ����������
(4)����I2�õ��ۣ���������Լ������ʣ�FeCl3���������ԣ��ɽ�I������ΪI2����Na2SO3����ǿ��ԭ�ԣ��ɽ�IO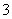 ��ԭΪI2��
��ԭΪI2��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и��������л�Ϊͬϵ�����
A��CH3��CH=CH2��CH3��CH2��CH=CH2
B��CH3��CH3��CH3��CH=CH2
C��CH3��CH2��CH3��CH3��CH=CH2
D��CH3��CH2��CH2��CH3��CH3��CH2��CHCl2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʯī�ڲ�����������ҪӦ�á�ij����ʯī�к�SiO2(7.8%)��Al2O3(5.1%)��Fe2O3(3.1%)��MgO(0.5%)�����ʡ���Ƶ��ᴿ���ۺ����ù������£�
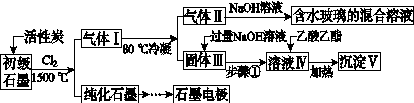
(ע��SiCl4�ķе�Ϊ57.6 �棬�����Ȼ���ķе������150 ��)
(1)��Ӧ����ͨ��Cl2ǰ����ͨһ��ʱ��N2����ҪĿ����____________________��
(2)���·�Ӧ��ʯī�����������ʾ�ת��Ϊ��Ӧ���Ȼ��������е�̼��������ҪΪ________�����������ij��õ�ˮ�����Ļ�ѧ��Ӧ����ʽΪ____________________________________________��
(3)�����Ϊ�����衢________��������Һ���е���������________��
(4)����Һ�����ɳ��������ܷ�Ӧ�����ӷ���ʽΪ______________________________________________��100 kg����ʯī�����ܻ�â�������Ϊ______kg��
(5)ʯī��������Ȼˮ����ͭ���ĵ绯ѧ�����������ͼ����ʾ��ͼ��������Ӧ��ע��
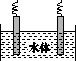
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
̼����ĺ���Ӱ��������ܡ�̼��������һ�ֲⶨ�����ǽ������е�̼����ת��Ϊ���壬���ò�̼������װ�ý��вⶨ��
(1)����װ��A���ڸ����½�x g�����е�̼����ת��ΪCO2��SO2��
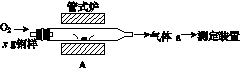
������a�ijɷ���______��
��������������FeS��ʽ���ڣ�A�з�Ӧ��
3FeS��5O2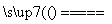 1________��3________��
1________��3________��
(2)������aͨ�����װ����(��ͼ)�����õζ����ⶨ��ĺ�����
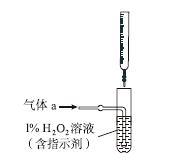
��H2O2����SO2�Ļ�ѧ����ʽ��__________________��
����NaOH��Һ�ζ����ɵ�H2SO4������z mL NaOH��Һ��������1 mL NaOH��Һ�൱���������Ϊy g����ø������������������________��
(3)������aͨ���̼װ����(��ͼ)�������������ⶨ̼�ĺ�����

������aͨ��B��C��Ŀ����________________________________________��
�ڼ��������̼������������Ӧ������������_______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������һ����Ҫ�Ļ�����Ʒ��ij��ȤС�����Ʊ���������ƾ���(Na2S2O3��5H2O)��
��.���������ϡ�
(1)Na2S2O3��5H2O����ɫ�����壬������ˮ����ϡ��Һ��BaCl2��Һ����������ɡ�
(2)��Na2CO3��Na2S�����Һ��ͨ��SO2���Ƶ�Na2S2O3�����ò�Ʒ����������Na2SO3��Na2SO4��
(3)Na2SO3�ױ�������BaSO3������ˮ��������ϡ���ᡣ
��.���Ʊ���Ʒ��
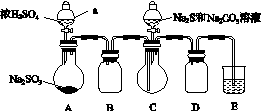
ʵ��װ����ͼ��ʾ(ʡ�Լг�װ��)��
ʵ�鲽�裺
(1)���װ�������ԣ���ͼʾ�����Լ���
����a��������________��E�е��Լ���________(ѡ��������ĸ���)��
A��ϡH2SO4
B��NaOH��Һ
C������NaHSO3��Һ
(2)����C����ƿ����Na2S��Na2CO3�Ļ����Һ������A����ƿ�μ�ŨH2SO4��
(3)��Na2S��Na2CO3��ȫ���ĺ�����Ӧ������C�еĻ�����Һ��________(��д��������)���ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ��
��.��̽���뷴˼��
(1)Ϊ��֤��Ʒ�к���Na2SO3��Na2SO4����С�����������ʵ�鷽�����뽫��������������(�����Լ���ϡHNO3��ϡH2SO4��ϡ���ᡢ����ˮ��ѡ��)
ȡ������Ʒ���ϡ��Һ���μ�����BaCl2��Һ���а�ɫ�������ɣ�____________________________________��������δ��ȫ�ܽ⣬���д̼�����ζ��������������ȷ����Ʒ�к���Na2SO3��Na2SO4��
(2)Ϊ����װ��C������Na2SO4�������ڲ��ı�ԭ��װ�õĻ����϶�ʵ�鲽��(2)�����˸Ľ����Ľ���IJ�����______________________________________________________________________________��
(3)Na2S2O3��5H2O���ܽ�����¶����������������ò�Ʒͨ��________�����ᴿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ��ʵ���Ҵ���ͼK352��(��)��ǩ���Լ�ƿ��ȡ�����ƽ���ȼ��ʵ�飬��ͼK352��(��)��

��������������(��)���������������� ��(��)
ͼK352
(1)װ��A������Ϊ________��ʵ���У��۲쵽�ĵ���ɫ�Ĺ���������________(д��ѧʽ)���۲쵽�Ļ�ɫ������________Ԫ�صķ�����ס�
(2)ʵ����ֻ���������ɫ�������ɡ�
�ӷ�Ӧ�Pʵ������²⣺�ú�ɫ���ʿ���Ϊ̼����һ����������ɵĻ���
��������������________��________(д��ѧʽ)��
(3)�Ժ�ɫ�������ʵ����������̽����
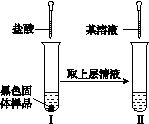
ͼK353
��ʵ����м��������Ŀ����________________________________________________________________________
________________________________________________________________________��
�ڽ�ͨ��ʵ��������IJ����ܿ���ȷ����ɫ���ʵ���ɣ������Ƹ���ơ�
[��ѡ�Լ���ϡ���ᡢKSCN��Һ��K3Fe(CN)6��Һ��10%��H2O2��Һ]
| ʵ����� | Ԥ�ڵ���������� | ��ػ�ѧ����ʽ |
| ȡ����ʵ����еij�����Һ������________ | ________________________________________________________________________ | ________________________________________________________________________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ϊ�������ݿ��ŵ��� ( )
A. ij��ˮ��pHΪ5��6
B��ij����ʯ��ˮ��Ũ����2��0 mol/l
C��ij�������ӵ�ֱ����160 nm
D��ij����������ܶ�Ϊ1��8 g��/cm3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ�У���Ӧ������������ص���
A������ͨ�����ȵ�Cu0��ĩ
B��������̼ͨ��Na202��ĩ
C������Fe203�������ȷ�Ӧ
D����п��Ͷ��Cu(N03)2��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±�Ϊij�л���ֱ�������Լ���Ӧ�������������л��������(����)
| �Լ� | �� | ���Ը��������Һ | NaHCO3��Һ |
| ���� | �ų����� | ��ɫ | ����Ӧ |
A��CH2===CH��COOH B��CH2===CHCH3
C��CH3COOCH2CH3 D��CH2===CHCH2OH
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com