
����Ŀ���±���Ԫ�����ڱ���һ���֣���֪��Ϊ������Ԫ�أ��䵥��Ϊ����ɫ���壬�ݱ��ش��й����⣺
�� | �� | ||||||
�� | �� | �� | �� | �� | |||
�� | �� |
(1)����Ԫ�آ��ԭ�ӽṹʾ��ͼ __________________
(2)����ЩԪ���У�����õķǽ���Ԫ���� ______�� ����õ�Ԫ����_____��дԪ�ط��� ����
(3)����ЩԪ�ص�����������Ӧˮ�����У� ������ǿ���� __________(д��ѧʽ)�������Ե�����������____________(д��ѧʽ)��д������֮�䷴Ӧ�����ӷ���ʽ�� ______________________________
��4���ڢ�����У���ѧ���ʽϻ��õ���________��дԪ�ط��� ����д��������֤�ý��۵�һ����ѧ��Ӧ��ʽ _________________________________________��
���𰸡� 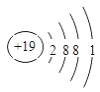 F Ar KOH Al��OH��3 Al��OH��3 +OH- =AlO2- + 2H2O Cl Cl2+2NaBr=Br2+2HCl
F Ar KOH Al��OH��3 Al��OH��3 +OH- =AlO2- + 2H2O Cl Cl2+2NaBr=Br2+2HCl
����������֪��Ϊ������Ԫ�أ��䵥��Ϊ����ɫ���壬�����S�����Ԣ���Li������F������Na������Al������Cl������Ar������K������Br��
(1)KԪ�ص�ԭ�ӽṹʾ��ͼΪ ��(2)����ЩԪ���У�����õķǽ���Ԫ����F�� ����õ�Ԫ����ϡ������Ar�� (3)����ЩԪ�ص�����������Ӧˮ�����У� ������ǿ����KOH�������Ե�����������Al(OH)3������֮�䷴Ӧ�����ӷ���ʽΪAl��OH��3 +OH- =AlO2- + 2H2O����4��ͬ������ԭ������������ǽ����Լ��������ڢ�����У���ѧ���ʽϻ��õ���Cl�������ܰ����û�����������֤�ý��ۣ���Ӧ�Ļ�ѧ��Ӧ��ʽΪ Cl2+2NaBr��Br2+2HCl��
��(2)����ЩԪ���У�����õķǽ���Ԫ����F�� ����õ�Ԫ����ϡ������Ar�� (3)����ЩԪ�ص�����������Ӧˮ�����У� ������ǿ����KOH�������Ե�����������Al(OH)3������֮�䷴Ӧ�����ӷ���ʽΪAl��OH��3 +OH- =AlO2- + 2H2O����4��ͬ������ԭ������������ǽ����Լ��������ڢ�����У���ѧ���ʽϻ��õ���Cl�������ܰ����û�����������֤�ý��ۣ���Ӧ�Ļ�ѧ��Ӧ��ʽΪ Cl2+2NaBr��Br2+2HCl��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ݱ�������ѧ���ѳɹ��ϳ���������O4���й�O4��˵����ȷ����
A. O4��Ħ��������64 g
B. ��ͬ������O4��O3����ԭ�Ӹ���֮��Ϊ1��1
C. O4��O2��Ϊͬλ��
D. O4��O3��O2������Ԫ�ص�ͬ���칹��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��ҵ�л�������������ˮ�������������������������ŷš�
��1����ҵ����NaHSO3��ԭ���������������£������Է�ˮ�м���NaHSO3ʹCr2O72- ��ԭ��ΪCr3+��Ȼ�������ʯ�ҵ��ڷ�ˮ��pH��ʹCr3+��ȫ������
�� д��NaHSO3��Cr2O72-��Ӧ�����ӷ���ʽ��__________________________________��
����֪25��ʱKsp[Cr(OH)3]=6.4��10-31������ȥ��ˮ��Cr3+��ʹ��Ũ��С��1��10-5 molL-1����ʱ��Һ�е�c(H+)<_________molL-1
��2����ˮ�и�Ԫ����Ũ�ȵIJⶨ�������£���һ������Cr2O72-��Cr3+�����Է�ˮ���м�������(NH4)2S2O8��Һ��Cr3+������Cr2O72-����г�ȥ������(NH4)2S2O8���ټ��������KI��Һ��Cr2O72-��I-��ȫ��Ӧ������Cr3+��I2���Ե���Ϊָʾ������Na2S2O3����Һ�ζ����յ㡣�ⶨ���������ʵ�ת����ϵ���£�Cr3+ ![]() Cr2O72-
Cr2O72- ![]() I2
I2 ![]() S4O62-
S4O62-
���������������У�������в�������ⶨ�ĸ�Ԫ����Ũ�Ȼ�________��ѡ�ƫ����ƫС�����䡱����
��ȷ��ȡ��Cr2O72-��Cr3+�����Է�ˮ��100.00 mL�������������ⶨ��ˮ���и�Ԫ����Ũ�ȣ�����0.01000 molL-1��Na2S2O3����Һ13.50 mL������÷�ˮ�и�Ԫ����Ũ�ȣ���mg��L-1��ʾ����д��������̡�_____________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵ���Ũ�ȵ�CuSO4��NaCl�������Ϻ���ʯī�缫���е�⣬�������У���ҺpH��ʱ��t�仯��������ͼ��ʾ��������˵���������
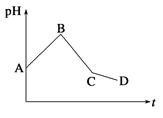
A������������Cl2��������O2�������Ȳ���Cu��������H2
B��AB������ֻ����Cl2������ֻ����Cu
C��BC�α�ʾ����������H���ŵ������H2
D��CD���൱�ڵ��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ��װ�û���������ȷ�����ܴﵽĿ�ĵ��ǣ� ��

A. ʵ���ʵ�����Ʊ����� B. ʵ����ö�����̼����Ȫʵ��
C. ʵ������к��ȵIJⶨ D. ʵ���������Ȼ�ͭ��Һ�õ�ͭ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Һ��ʯ������Ϊȼ�������ձ������м�ͥ�����Ǻ����������ʵĻ�������ڳ�ѹ������Щ���ʵķе����±���ʾ��
�������� | ���� | ���� | ���� | ���� | ���� |
�е�/�� | ��88.6 | ��42.1 | ��0.5 | 36.1 | 69.2 |
�ڳ�����ʹ����������ų�ʱ����ƿ�г�ʣ��һЩҺ̬��������Щ�������п�����(����)
A. ���顢����Ͷ��� B. ����ͱ���
C. ֻ������ D. ����ͼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��SO32��+I2+H2O= SO42��+2I��+2H����ij��ɫ��Һ�п��ܺ���I����NH4����Cu2����SO32���������Һ�м���������ˮ����Һ�Գ���ɫ�����й�����Һ��ɵ��ж���ȷ����
�ٿ϶�����I�� �ڿ϶�����Cu2�� �ۿ϶�����SO32 �� �ܿ��ܺ���I��
A. �٢� B. �٢ڢ� C. �٢� D. �ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
�л���A��K������ת����ϵ��E��H����FeCl3��Һ������ɫ��Ӧ��Iת���IJ�Kֻ��һ�ֽṹ����K��ʹ���CCl4��Һ��ɫ��

��֪���� D��������˴Ź�������ͼ���£�
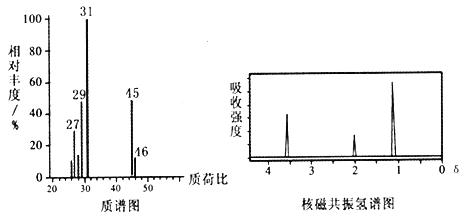
�� �����ǻ�ͬʱ����ͬһ̼ԭ���ϵĽṹ�Dz��ȶ��ģ�����������ˮ��Ӧ��CH3CH(OH)2��CH3CHO + H2O��
��ش��������⣺
��1��C�й����ŵ�������_______��H��I�ķ�Ӧ������__________��
��2��K�Ľṹ��ʽΪ________������aΪ______��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
��F��G_________��
��A��B + E__________________��
��4��L����Է���������H��14��ͬϵ�ͬʱ������������L ��ͬ���칹����____�֡�
a.��FeCl3��Һ����ɫ��Ӧ b������NaHCO3��Һ������Ӧ
���б����ϵ�һ�����ֻ�����ֵ�L�Ľṹ��ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ϳ�ҩ��X ��·�����£�
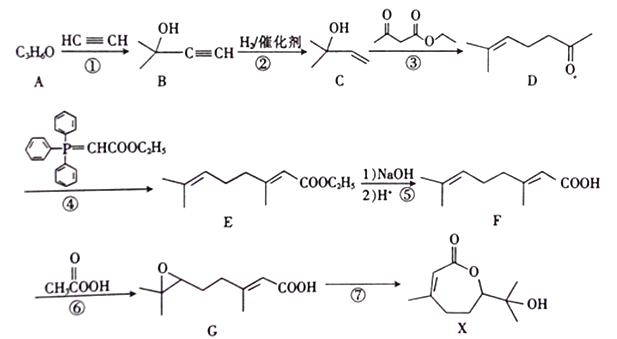
��1��X�к��������ŵ�����Ϊ________��A�ĺ˴Ź�������ֻ��һ�����շ壬A�Ľṹ��ʽΪ____________��
��2����Ӧ�ٵķ�Ӧ����Ϊ_________����Ӧ�ٻ���õ���һ����H�������ʽΪC8H14O2��д��H�Ľṹ��ʽ��______________��
��3����Ӧ�IJ���ΪG��_________����ṹ��ʽ����
��4��X������ȥһ����ˮ�ɵ�����J��ͬʱ��������������J��ͬ���칹�干��_____�֡����к˴Ź�������ֻ���������շ��ͬ���칹��������Ũ��ˮ��Ӧ�Ļ�ѧ����ʽΪ__________��
a.����FeCl3��Һ������ɫ��Ӧ
b���ܷ�����ȥ��Ӧ
c��������ֻ��2��ȡ�������ҷ�����ֻ��2����
��5�����������ϳ�·�ߣ����һ����CH3OH��CH��CHΪԭ���Ʊ��۱�ϩ��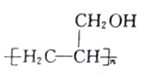 �ĺϳ�·��ͼ�����Լ����ã����ϳ�·��ʾ�����£�_______________
�ĺϳ�·��ͼ�����Լ����ã����ϳ�·��ʾ�����£�_______________
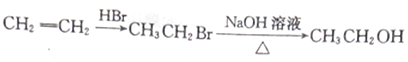
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com