
| ���� | ���� | ԭú ����Ҫ�ɷ���C�� | ���� ����Ҫ�ɷ�C8H18�� |
| ������kJ�� | 285.8 | 250.9 | 4910 |
���� ��1��1molH2��ȫȼ�շų�������Ϊ��285.8kJ�������Ȼ�ѧ����ʽ��2H2��g��+O2��g��=2H2O ��l����H=-571.6 kJ/mol��
��2��H2ȼ�շų�����������ԭú�����ͣ��Ҳ�������Ⱦ��
��3����ⱥ��ʳ��ˮ�����ݵ缫��Ӧ�ж����ɲ����д���ӷ���ʽ��
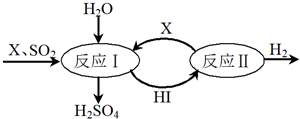
����ͼ��֪��Ӧ���ǵⵥ�����������Ӧ�������������ᣬ��Ӧ���������ֽ����������͵ⵥ�ʣ��ɴ˷������
��1���ù��̿�ѭ�����õ�������I2��HI��
��2���ܷ�Ӧ�Ļ�ѧ����ʽ�ǣ�SO2+2H2O=H2SO4+H2��
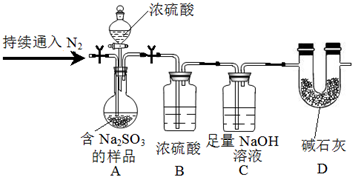
��3����װ��B��Ũ���ᣬ����������������ˮ������
����ʵ��ǰ��װ��C��������Ϊng������Ԫ���غ�����������������õ�����������
��� �⣺��1��1molH2��ȫȼ�շų�������Ϊ��285.8kJ�������Ȼ�ѧ����ʽ��2H2��g��+O2��g��=2H2O ��l����H=-571.6 kJ/mol���ʴ�Ϊ��2H2��g��+O2��g��=2H2O ��l����H=-571.6 kJ/mol��
��2��H2ȼ�շų�����������ԭú�����ͣ��Ҳ�������Ⱦ���ʴ�Ϊ����ͬ�����£���������H2ȼ�շų�����������ԭú�����ͣ���������Ⱦ����ѭ�����ã�
��3����ⱥ��ʳ��ˮ����Һ�е�������������ʧ���������������������������õ���������������������������������Ũ�����������������ƣ���Ӧ�����ӷ���ʽ��2Cl-+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2OH-+H2��+Cl2�����ʴ�Ϊ��2Cl-+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2OH-+H2��+Cl2����
����ͼ��֪��Ӧ���ǵⵥ�����������Ӧ�������������ᣬ��Ӧ���������ֽ����������͵ⵥ�ʣ�
��1���ù��̿�ѭ�����õ�������I2��HI���ʴ�Ϊ��I2��HI��
��2���ܷ�Ӧ�Ļ�ѧ����ʽ�ǣ�SO2+2H2O=H2SO4+H2���ʴ�Ϊ��SO2+2H2O=H2SO4+H2��
��3����װ��B��Ũ���ᣬ����������������ˮ�������ʴ�Ϊ������SO2��
����ʵ��ǰ��װ��C��������Ϊng������Ԫ���غ�����������������õ�������������������Ϊ����Na2SO3����Ʒ������װ��Cͨ��SO2 ǰ����������ʴ�Ϊ����Na2SO3����Ʒ������װ��Cͨ��SO2 ǰ���������
���� ���⿼�����Ȼ�ѧ����ʽ����д���缫��Ӧʽ����д�����ʳɷֵ�ʵ����Ʒ�����ʵ����֤�ķ���Ӧ�ã���Ҫ��ʵ������������������ʵķ���Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�


 �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д� �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ͨ��״���£����Ƶ���ˮ�ʻ���ɫ | |
| B�� | ��������ˮ���뵽��ɫʯ����Һ�У���Һֻ�ܱ�ɺ�ɫ | |
| C�� | ����ˮ���뵽NaHCO3��ĩ�У������ݲ��� | |
| D�� | ����ˮ���뵽�����ữ��AgNO3��Һ�У��а�ɫ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2OͶ��ˮ�� | B�� | Na2O2Ͷ��ˮ�� | ||
| C�� | AlͶ��NaOH��Һ�� | D�� | ����С�մ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�������չ� | B�����ʯ�� | C�� ˮ����� | D��Ԥ������ |
 |  |  |  |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.5 mol/��L•min�� | B�� | 2mol/��L•s�� | C�� | 2mol/��L•min�� | D�� | 3mol/��L•min�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2NA | B�� | NA | C�� | ����NA | D�� | 6.02��1022 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
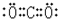 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ţ�̾���ҵ���ͺ�õ������� | B�� | �ù�ҵʳ�����Ƶ��ݲ� | ||
| C�� | ù��Ĵ��ס����� | D�� | �ü�ȩ��Һ���ݵĺ���Ʒ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com