
| ||
| �� |
| ||
| �� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||

| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| �� |
| ||
| �� |
| 2016��6 |
| 2688��5 |
| 2016��6 |
| 2688��5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
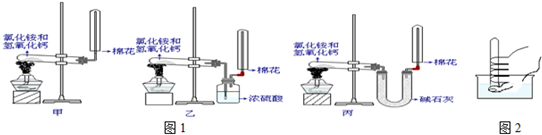
ij����С����ʵ�����Ʊ��������������йذ�������;�����ʵ�̽����
��1��д��ʵ������ȡ�����Ļ�ѧ����ʽ�� ��
��2���ϳɰ��Ի�ѧ������ҵ������Ҫ���塣д������������Ҫ��;��
�� �� ��
��3����С��ͬѧ�����ͼ��ʾװ��̽�������Ļ�ԭ�ԡ�
�ٰ��������Ļ�ѧ����ʽΪ ��
����ʵ��ʱͨ�백�������ʹ��죬����ƿ�л���ְ��̣������ʵĻ�ѧʽΪ ��
��4����С��ͬѧ�������Ͷ�����̼Ϊԭ���Ʊ������ϴ�����̼�����Һ������¼� ����������
�ټ������Ƚ�������̼ͨ��ˮ�У�����ܽ����ͨ�백����
�ҷ������Ƚ�����ͨ��ˮ�У�����ܽ����ͨ�������̼��
�����ķ����ǣ� �������� ��
�ڼ����������NH4+�ķ���Ϊ ��
���������ʾʽд����ˮ�д��ڵ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
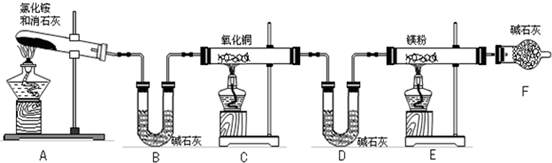
ijѧ����������װ��̽�������백��֮��ķ�Ӧ������CΪ��������������백����Ӧ��װ�á�
��ش��������⣺
��1��װ��A�е���ƿ�ڹ��岻��ѡ��
A����ʯ�� B����ʯ�� C������������ D���ռ�
��2��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ
��3��Bװ�õ����� ��Eװ�õ�����
��4��װ��C�ڳ���Ũ��İ��̲��������ڱ����ᣬ��һ�������ǿ�������Ҫ�ɷ�֮һ��д����Ӧ�Ļ�ѧ����ʽ��
��5����װ��C��G���ݳ���β���п��ܺ��л���ɫ���ж����壬��Ҫ������
д�����ӷ���ʽ
ʵ�����Ʊ����������з���������ѡ�õ��� ��
�� ��̬�Ȼ�識��ȷֽ� �� Ũ��ˮ�м��������������
�� ����Ũ��ˮ �� ��̬�Ȼ�����������ƻ�ϼ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com