
£Ø14·Ö£©
£Ø1£©“æĖ®ÖŠc(H£«)£½5.0”Į10£7mol/L£¬Ōņ“ĖŹ±“æĖ®ÖŠµÄc(OH£)£½ £»
ČōĪĀ¶Č²»±ä£¬µĪČėĻ”ĮņĖįŹ¹c(H£«)£½5.0”Į10£3mol/L£¬Ōņc(OH£)£½ ”£
£Ø2£©ŌŚCH3COONaµÄČÜŅŗÖŠ£¬ø÷Ąė×ÓµÄĪļÖŹµÄĮæÅضČÓɓ󵽊”µÄĖ³ŠņŹĒ ”£
£Ø3£©Ć÷·ÆµÄĖ®ČÜŅŗĻŌĖįŠŌ£¬ĘäŌŅņŹĒ£ØÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£© ”£
£Ø4£©ŌŚ25”ę”¢101kPaĻĀ£¬0.5molµÄ¼×“¼£ØCH3OH£©ĶźČ«Č¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬Ė®Ź±·Å³ö352kJµÄČČĮ棬Ōņ±ķŹ¾¼×“¼Č¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø5£©1L 1 mol”¤L£1 H2SO4ČÜŅŗÓė2L 1 mol”¤L£1 NaOHČÜŅŗĶźČ«·“Ó¦£¬·Å³ö114.6kJČČĮ棬Ōņ±ķŹ¾ÖŠŗĶČȵÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø6£©ŅŃÖŖ£ŗ¢Ł N2(g)+2O2(g)£½2NO2(g) ”÷H£½+67.7 kJ/mol £»
¢Ś N2H4(g)+O2(g)£½N2(g)+2H2O(g) ”÷H£½£534 kJ/mol
ŌņN2H4ÓėNO2ÓėĶźČ«·“Ӧɜ³ÉµŖĘųŗĶĘųĢ¬Ė®µÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø1£©5.0”Į10-7mol/L (2·Ö) 5.0”Į10-11mol/L(2·Ö)
£Ø2£©c( Na+) > c(CH3COO£) > c( OH£)> c(H+)£Ø2·Ö£©£¬
£Ø3£©Al3+ + 3H2O  Al(OH)3 + 3H+£Ø£²·Ö£© Al(OH)3£»
Al(OH)3 + 3H+£Ø£²·Ö£© Al(OH)3£»
£Ø4£©CH3OH (l)£«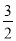 O2 (g) £½CO2 (g)£«2H2O (l) ¦¤H£½£704 kJ”¤mol-1
O2 (g) £½CO2 (g)£«2H2O (l) ¦¤H£½£704 kJ”¤mol-1
£Ø5£©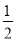 H2SO4(aq)£«NaOH(aq)£½
H2SO4(aq)£«NaOH(aq)£½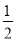 Na2SO4(aq)£«H2O(l) ¦¤H£½£57.3 kJ”¤mol-1£Ø£²·Ö£©
Na2SO4(aq)£«H2O(l) ¦¤H£½£57.3 kJ”¤mol-1£Ø£²·Ö£©
£Ø6£©2 N2H4 (g) + 2NO2 (g) £½ 3N2 (g) + 4H2O (g) ¦¤H£½£1135.7 kJ”¤mol-1 £Ø£²·Ö£©
»ņŠ“³É 2N2H4 (g) + NO2 (g) £½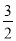 N2 (g) + £²H2O (g) ¦¤H£½”Ŗ567.85 kJ”¤mol-1
N2 (g) + £²H2O (g) ¦¤H£½”Ŗ567.85 kJ”¤mol-1
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ£Ø1£©ŌŚ“æĖ®ÖŠ“ęŌŚµēĄėĘ½ŗā£ŗH2O H£«£«OH£,“æĖ®ÖŠc(H£«)£½5.0”Į10£7mol/L£¬Ōņ“ĖŹ±“æĖ®ÖŠµÄc(OH£)£½5.0”Į10£7mol/L. Kw=2.5”Į10£13;ČōĪĀ¶Č²»±ä£¬µĪČėĻ”ĮņĖįŹ¹c(H£«)£½5.0”Į10£3mol/L£¬Ōņc(OH£)£½Kw”Āc(H£«)£½2.5”Į10£13”Ā5.0”Į10£3=5.0”Į10£11mol/L£»£Ø2£©ŌŚCH3COONaµÄČÜŅŗÖŠ£¬ÓÉÓŚCH3COO£Ė®½āĻūŗÄ£¬ĖłŅŌc( Na+) > c(CH3COO£)£»Ė®½āĻūŗÄĖ®µēĄė²śÉśµÄH+£¬ĘĘ»µĮĖĖ®µÄµēĄėĘ½ŗā£¬×īÖÕŹ¹ČÜŅŗÖŠµÄOH-µÄÅØ¶Č“óÓŚH+µÄÅØ¶Č£¬c( OH£)> c(H+)”£µ«ŹĒŃĪµÄĖ®½ā³Ģ¶ČŹĒĪ¢ČõµÄ£¬ŃĪµēĄė²śÉśµÄĄė×ÓÅضČŌ¶“óÓŚĖ®µēĄė²śÉśµÄĄė×ÓµÄÅØ¶Č£¬ĖłŅŌc(CH3COO£) > c( OH£) c(H+)”£¹Źø÷Ąė×ÓµÄĪļÖŹµÄĮæÅضČÓɓ󵽊”µÄĖ³ŠņŹĒc( Na+) > c(CH3COO£) > c( OH£)> c(H+)£»£Ø3£©Ć÷·ÆµÄĖ®ČÜŅŗĻŌĖįŠŌ£¬ĘäŌŅņŹĒAl3+ + 3H2O
H£«£«OH£,“æĖ®ÖŠc(H£«)£½5.0”Į10£7mol/L£¬Ōņ“ĖŹ±“æĖ®ÖŠµÄc(OH£)£½5.0”Į10£7mol/L. Kw=2.5”Į10£13;ČōĪĀ¶Č²»±ä£¬µĪČėĻ”ĮņĖįŹ¹c(H£«)£½5.0”Į10£3mol/L£¬Ōņc(OH£)£½Kw”Āc(H£«)£½2.5”Į10£13”Ā5.0”Į10£3=5.0”Į10£11mol/L£»£Ø2£©ŌŚCH3COONaµÄČÜŅŗÖŠ£¬ÓÉÓŚCH3COO£Ė®½āĻūŗÄ£¬ĖłŅŌc( Na+) > c(CH3COO£)£»Ė®½āĻūŗÄĖ®µēĄė²śÉśµÄH+£¬ĘĘ»µĮĖĖ®µÄµēĄėĘ½ŗā£¬×īÖÕŹ¹ČÜŅŗÖŠµÄOH-µÄÅØ¶Č“óÓŚH+µÄÅØ¶Č£¬c( OH£)> c(H+)”£µ«ŹĒŃĪµÄĖ®½ā³Ģ¶ČŹĒĪ¢ČõµÄ£¬ŃĪµēĄė²śÉśµÄĄė×ÓÅضČŌ¶“óÓŚĖ®µēĄė²śÉśµÄĄė×ÓµÄÅØ¶Č£¬ĖłŅŌc(CH3COO£) > c( OH£) c(H+)”£¹Źø÷Ąė×ÓµÄĪļÖŹµÄĮæÅضČÓɓ󵽊”µÄĖ³ŠņŹĒc( Na+) > c(CH3COO£) > c( OH£)> c(H+)£»£Ø3£©Ć÷·ÆµÄĖ®ČÜŅŗĻŌĖįŠŌ£¬ĘäŌŅņŹĒAl3+ + 3H2O  Al(OH)3 + 3H+£»£Ø4£©Č¼ÉÕČČŹĒ1molµÄæÉČ¼ĪļĶźČ«Č¼ÉÕ²śÉśĪČ¶ØµÄŃõ»ÆĪļŹ±Ėł·Å³öµÄČČĮ棬øł¾ŻĢāŅāæÉµĆ¼×“¼Č¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½ĪŖCH3OH (l)£«
Al(OH)3 + 3H+£»£Ø4£©Č¼ÉÕČČŹĒ1molµÄæÉČ¼ĪļĶźČ«Č¼ÉÕ²śÉśĪČ¶ØµÄŃõ»ÆĪļŹ±Ėł·Å³öµÄČČĮ棬øł¾ŻĢāŅāæÉµĆ¼×“¼Č¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½ĪŖCH3OH (l)£«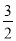 O2 (g)£½CO2 (g)£«2H2O (l) ¦¤H£½£704 kJ”¤mol-1£»£Ø5£©ÖŠŗĶČČŹĒĖį¼ī·¢ÉśÖŠŗĶ·“Ó¦²śÉś1molµÄĖ®Ź±Ėł·Å³öµÄČČĮ棬ÓÉÓŚ1L 1 mol”¤L£1 H2SO4ČÜŅŗÓė2L 1 mol”¤L£1 NaOHČÜŅŗĶźČ«·“Ó¦£¬·Å³ö114.6kJČČĮ棬Ōņ±ķŹ¾ÖŠŗĶČȵÄČČ»Æѧ·½³ĢŹ½ĪŖ
O2 (g)£½CO2 (g)£«2H2O (l) ¦¤H£½£704 kJ”¤mol-1£»£Ø5£©ÖŠŗĶČČŹĒĖį¼ī·¢ÉśÖŠŗĶ·“Ó¦²śÉś1molµÄĖ®Ź±Ėł·Å³öµÄČČĮ棬ÓÉÓŚ1L 1 mol”¤L£1 H2SO4ČÜŅŗÓė2L 1 mol”¤L£1 NaOHČÜŅŗĶźČ«·“Ó¦£¬·Å³ö114.6kJČČĮ棬Ōņ±ķŹ¾ÖŠŗĶČȵÄČČ»Æѧ·½³ĢŹ½ĪŖ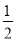 H2SO4(aq)£«NaOH(aq)£½
H2SO4(aq)£«NaOH(aq)£½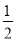 Na2SO4(aq)£«H2O(l) ¦¤H£½£57.3 kJ”¤mol-1£»£Ø6£©¢Ś”Į2£¢Ł£¬ÕūĄķæɵĆ2 N2H4 (g) + 2NO2 (g) £½ 3N2 (g) + 4H2O (g) ¦¤H£½£1135.7 kJ”¤mol-1»ņŠ“ĪŖ2N2H4 (g) + NO2 (g) £½
Na2SO4(aq)£«H2O(l) ¦¤H£½£57.3 kJ”¤mol-1£»£Ø6£©¢Ś”Į2£¢Ł£¬ÕūĄķæɵĆ2 N2H4 (g) + 2NO2 (g) £½ 3N2 (g) + 4H2O (g) ¦¤H£½£1135.7 kJ”¤mol-1»ņŠ“ĪŖ2N2H4 (g) + NO2 (g) £½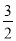 N2 (g) + £²H2O (g) ¦¤H£½”Ŗ567.85 kJ”¤mol-1”£
N2 (g) + £²H2O (g) ¦¤H£½”Ŗ567.85 kJ”¤mol-1”£
æ¼µć£ŗæ¼²éČõµē½āÖŹµÄµēĄė”¢Ė®µÄĄė×Ó»ż³£Źż”¢ŃĪµÄĖ®½ā”¢øĒĖ¹¶ØĀɵÄÓ¦ÓĆ”¢Ąė×ÓÅØ¶Č±Č½Ļ”¢ÖŠŗĶČČ”¢Č¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½µÄŹéŠ“µÄÖŖŹ¶”£


 ÄÜæ¼ŹŌĘŚÄ©³å“Ģ¾ķĻµĮŠ“š°ø
ÄÜæ¼ŹŌĘŚÄ©³å“Ģ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014¼ŖĮÖŹ”³¤“ŗŹŠø߶žĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ijŃõ»ÆĪļµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖa£¬ĘäĻąĶ¬¼ŪĢ¬µÄĮņĖįŃĪµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖb£¬ŌņøĆŌŖĖŲµÄ»ÆŗĻ¼ŪµÄŹżÖµĪŖ£ŗ
A £®(b-a)/20 B£®(b-a)/40 C£®(a -b)/80 D£®(a-b)/20
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014¼ŖĮÖŹ”³¤“ŗŹŠøßŅ»ĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠĶ¼Ź¾ÖŠ¹ŲÓŚĶµē¼«µÄĮ¬½Ó“ķĪóµÄŹĒ
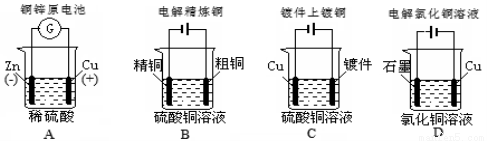
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014¼ŖĮÖŹ”°×³ĒŹŠø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»Æѧ£ØB¾ķ£©ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ijĢ½¾æŠ”×éŌŚÄ³ĪĀ¶ČĻĀ²ā¶ØČÜŅŗµÄpHŹ±·¢ĻÖ£ŗ0.01mol”¤L£1µÄNaOHČÜŅŗÖŠ£¬ÓÉĖ®µēĄė³öµÄc(H+)”¤c(OH£)=10£22£¬ŌņøĆŠ”×éŌŚøĆĪĀ¶ČĻĀ²āµĆ0.1mol”¤L£1NaOHČÜŅŗµÄpHÓ¦ĪŖ
A£®13 B£®12 C£®11 D£®10
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014¼ŖĮÖŹ”°×³ĒŹŠø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»Æѧ£ØB¾ķ£©ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
Ä³Ń§ÉśÓūĶź³É·“Ó¦2HCl£«2Ag== 2AgCl”ż£«H2”ü¶ųÉč¼ĘĮĖĻĀĮŠĖÄøöŹµŃ飬ÄćČĻĪŖæÉŠŠµÄŹĒ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014¼ŖĮÖŹ”°×³ĒŹŠø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£ØA¾ķ£©£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ŌŚČŻ»żĪŖVLµÄĆܱÕČŻĘ÷ÖŠ·ÅČė2LAŗĶ1LB£¬3A(g)+B(g)  nC(g)+2D(g)£¬“ļµ½Ę½ŗāŗó£¬AĪļÖŹµÄĮæ¼õÉŁ1/2£¬»ģŗĻĘųĢåµÄĘ½¾łÄ¦¶ūÖŹĮæŌö“ó1/8£¬ŌņøĆ·“Ó¦ÖŠnµÄÖµ£Ø £©
nC(g)+2D(g)£¬“ļµ½Ę½ŗāŗó£¬AĪļÖŹµÄĮæ¼õÉŁ1/2£¬»ģŗĻĘųĢåµÄĘ½¾łÄ¦¶ūÖŹĮæŌö“ó1/8£¬ŌņøĆ·“Ó¦ÖŠnµÄÖµ£Ø £©
A£® 1 B£® 2 C£® 3 D£® 4
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014¼ŖĮÖŹ”°×³ĒŹŠø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£ØA¾ķ£©£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
25”ꏱ£¬Ė®µÄµēĄė“ļµ½Ę½ŗā£ŗH2O H£«£«OH££¬ĻĀĮŠŠšŹöÕżČ·µÄŹĒ£Ø £©
H£«£«OH££¬ĻĀĮŠŠšŹöÕżČ·µÄŹĒ£Ø £©
A£®ĻņĖ®ÖŠ¼ÓČė°±Ė®£¬Ę½ŗāÄęĻņŅĘ¶Æ£¬c(OH£)½µµĶ
B£®ĻņĖ®ÖŠ¼ÓČėÉŁĮæ¹ĢĢåĮņĖįĒāÄĘ£¬c(H£«)Ōö“ó£¬KW²»±ä
C£®ĻņĖ®ÖŠ¼ÓČėÉŁĮæ¹ĢĢåCH3COONa£¬Ę½ŗāÄęĻņŅĘ¶Æ£¬c(H£«)½µµĶ
D£®½«Ė®¼ÓČČ£¬KWŌö“ó£¬pH²»±ä
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014¼ŖĮÖŹ”°×³ĒŹŠøßŅ»ÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»Æѧ£ØB¾ķ£©ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
(14·Ö)ÓŠČżÖÖ½šŹōµ„ÖŹA”¢B”¢C£¬ĘäÖŠAµÄŃęÉ«·“Ó¦ĪŖ»ĘÉ«£¬B”¢CŹĒ³£¼ū½šŹō”£ČżÖÖ½šŹōµ„ÖŹA”¢B”¢CÄÜÓėĘųĢå¼×”¢ŅŅ”¢±ū¼°ĪļÖŹD”¢E”¢F”¢G”¢HÖ®¼ä·¢ÉśČēĻĀ×Ŗ»Æ¹ŲĻµ£ØĶ¼ÖŠÓŠŠ©·“Ó¦µÄ²śĪļŗĶ·“Ó¦µÄĢõ¼žĆ»ÓŠ±ź³ö£©”£
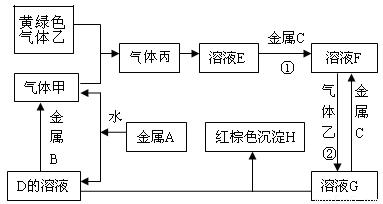
Ēėøł¾ŻŅŌÉĻŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öĻĀĮŠĪļÖŹµÄ»ÆѧŹ½£ŗ
A_____________£»H ______________£» G___________£»ŅŅ____________”£
£Ø2£©Š“³öĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
·“Ó¦¢Ł________________________________£»·“Ó¦¢Ś_________________________________”£
£Ø3£© ·“Ó¦¢ŚÖŠµē×Ó×ŖŅʵďżÄæĪŖ6.02”Į1023øöŹ±ĻūŗĵÄĘųĢåŅŅµÄĪļÖŹµÄĮæĪŖ mol
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014¼ŖĮÖŹ”°×³ĒŹŠøßŅ»ÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»Æѧ£ØA¾ķ£©ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ÅäÖĘŅ»¶ØĢå»żŅ»¶ØÅØ¶ČµÄNaOHČÜŅŗŹ±£¬ĻĀĮŠ²Ł×÷µ¼ÖĀ½į¹ūĘ«øߵďĒ£Ø £©
A£®³ĘĮæNaOH¹ĢĢåŹ±¶Æ×÷»ŗĀż B£®¹ĢĢåČܽāĶźĪ“ĄäČ“¾Ķ×ŖŅĘÖĮČŻĮæĘæ
C£®¶ØČŻŗóÕńµ“·¢ĻÖŅŗĆęµĶÓŚæĢ¶ČĻß¾Ķ²»¹ÜĮĖ D£®ÓŠÉŁĮæŅŗĢ彦³ö
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com