
 Ė®¹ø£¬³£ÓƵķ½·ØŹĒĻņĘæÖŠ¼ÓČėĻ”“×Ėį½«ĘäČܽā£¬Ęä·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ¢Ł
Ė®¹ø£¬³£ÓƵķ½·ØŹĒĻņĘæÖŠ¼ÓČėĻ”“×Ėį½«ĘäČܽā£¬Ęä·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ¢Ł 

 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ŌŚĻ”°±Ė®ÖŠĶØČė¹żĮæCO2£ŗNH3”¤H2O+CO2”śNH4++HCO3£ |
| B£®Ļņ“ĪĀČĖįÄĘČÜŅŗÖŠĶØČėÉŁĮæµÄCO2£ŗ2ClO£+H2O+CO2”ś2HClO+CO32£ |
| C£®ÓĆĖ«ŃõĖ®ŗĶĻ”ĮņĖį“¦ĄķÓ”Ė¢µēĀ·°å£ŗCu+H2O2£«2H+”śCu2++2H2O |
| D£®NH4HSO4ČÜŅŗÖŠ¼ÓČė×ćĮæBa(OH)2ČÜŅŗ£ŗH++SO42£+Ba2++OH£”śBaSO4”ż+H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Ģ¼ĖįĒāļ§ČÜŅŗÓė×ćĮæĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦ + OH£NH3”¤H2O |
| B£®AlCl3ČÜŅŗÖŠ¼ÓČė×ćĮæµÄ°±Ė®£ŗAl3++ 4NH3?H2OØTØTAlO2£+4NH4++2H2O |
| C£®ĻņĆ÷·ÆČÜŅŗÖŠÖšµĪ¼ÓČėBa(OH)2ČÜŅŗÖĮSO42-Ē”ŗĆ³ĮµķĶźČ« 2Al3£«+ 3SO42-+ 3Ba2£«+ 6OH£2Al(OH)3”ż+ 3BaSO4”ż |
| D£®FeCl2ČÜŅŗøśCl2·“Ó¦£ŗ2Fe2++Cl2=2Fe3++2Cl£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ŹÆ»ŅŹÆČÜÓŚŃĪĖį£ŗCO32- + 2H+="=" H2O +CO2”ü |
| B£®Al2O3·ŪÄ©ČÜÓŚNaOHČÜŅŗÖŠ£ŗAl2O3+2OH-=2AlO2-+H2O |
| C£®ĮņĖįĒāÄĘČÜŅŗÓėĒāŃõ»Æ±µČÜŅŗµÄ·“Ó¦£ŗBa2++SO42-£½BaSO4”ż |
| D£®Ńõ»ÆÄĘ¼ÓČėŃĪĖįÖŠ£ŗO2££«2H£«===H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
A£®CuCl2£«2NaOH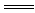 Cu(OH)2”ż£«2NaCl Cu(OH)2”ż£«2NaClCu2£«£«2OH£ 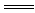 Cu(OH)2”ż Cu(OH)2”ż |
B£®BaCO3£«2HCl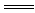 BaCl2£«CO2”üŹ®H2O BaCl2£«CO2”üŹ®H2OCO 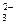 Ź®2H£« Ź®2H£«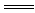 CO2”üŹ®H2O CO2”üŹ®H2O |
C£®Ca(NO3)2£«Na2CO3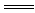 CaCO3”ż£«2NaNO3 CaCO3”ż£«2NaNO3Ca2£«£«CO 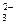 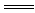 CaCO3”ż CaCO3”ż |
| D£®2KOH£«H2SO4£½K2SO4Ź®2H2O |
 H2O
H2O²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®16O2Óė18O2»„ĪŖĶ¬·ÖŅģ¹¹Ģå |
| B£®LiŌŚŃõĘųÖŠČ¼ÉÕÖ÷ŅŖÉś³ÉLi2O2 |
| C£®Br2ČÜÓŚĖ®ÖʱøäåĖ®µÄĄė×Ó·½³ĢŹ½ŹĒ£ŗBr2 + H2O = Br£+ BrO£+ 2H£« |
| D£®pH=1µÄČÜŅŗÖŠ£¬K£«”¢Na£«”¢NO3£”¢S2O32£²»ÄÜ“óĮæ¹²“ę |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ĒāŃõ»Æ±µÓėŃĪĖį·“Ó¦£ŗ OH”Ŗ+H+=H2O |
| B£®FeŗĶŃĪĖį·“Ó¦£ŗ2Fe£«6H+£½2Fe3+£«3H2”ü |
| C£®CuŗĶAgNO3ČÜŅŗ·“Ó¦£ŗCu£«Ag+£½Cu2+£«Ag |
| D£®ŹÆ»ŅŹÆŗĶŃĪĖį·“Ó¦£ŗCO32”Ŗ£«2H+£½CO2”ü£«H2O |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com