


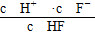 ��
��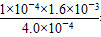 ��4��10��4
��4��10��4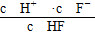 ���ɡ�(3)��pH��4ʱ����Һ�е�c(F��)��1.6��10��3 mol��L��1����Һ��c(Ca2��)��2.0��10��4 mol��L��1����Һ�����ӻ�Qc��c(Ca2��)��c2(F��)��5.12��10��10��Ksp(CaF2)��1.5��10��10�����г���������
���ɡ�(3)��pH��4ʱ����Һ�е�c(F��)��1.6��10��3 mol��L��1����Һ��c(Ca2��)��2.0��10��4 mol��L��1����Һ�����ӻ�Qc��c(Ca2��)��c2(F��)��5.12��10��10��Ksp(CaF2)��1.5��10��10�����г���������


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ��ѧʽ | CH3COOH | H2CO3 | HClO |
| ����ƽ �ⳣ�� | K��1.8��10��5 | K1��4.3��10��7 K2��5.6��10��11 | K��3.0��10��8 |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NaOH | B��H2SO4 | C��NaCl | D��Na2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���к�HCI�Ķ� | B���к�CH3COOH�Ķ� | C����ͬ | D�����Ƚ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 H����H2PO3����
H����H2PO3����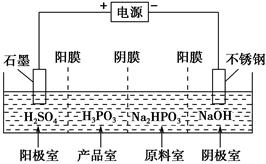
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ��ѧʽ | CH3COOH | H2CO3 | HClO | |
| ����ƽ�ⳣ�� | Ka��1.8��10��5 | Kal��4.3��10��7 | Ka2��5.6��10��11 | Ka��3.0��10��8 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ��ѧʽ | ���볣�� |
| HClO | K=3��10-8 |
| H2CO3 | K1=4��10-7 |
| K2=6��10-11 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������£���0.1 mol/L HX��Һ��pH����pH��1��֤��HX������ |
| B�������£���1 mol/L NaX��Һ��pH����pH��7��֤��HX������ |
| C����ͬ�����£���0.1 mol/L��HCl��0.1 mol/L��HX���е�����ʵ�飬����HX��Һ�മ���ĵ��ݽϰ���֤��HXΪ���� |
| D������Ũ�ȡ��������HCl��NaX��Һ��ϣ��������ҺpH<7��֤��HX������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com