
ij��ѧ��ȤС��������װ��,�Ի������Ʊ�����ϩ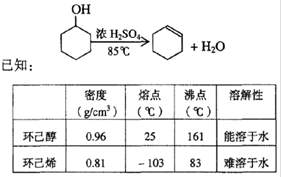
(1)�Ʊ���Ʒ
��12.5mL�����������Թ�A�У��ټ���lmLŨ���ᣬҡ�Ⱥ�������Ƭ����������
����Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
�ٵ���B���˵�������е�������____________��
���Թ�C���ڱ�ˮԡ�е�Ŀ����________________��
��2)�Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��_________��(���ϻ���)����Һ����_________ (������)ϴ�ӡ�
a��KMnO4��Һ b��ϡH2SO4 c��Na2CO3��Һ
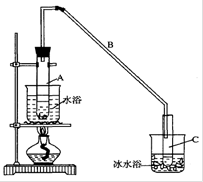
���ٽ�����ϩ��ͼװ��������ȴˮ ��_________�ڽ��롣����ʱҪ������ʯ�ң�Ŀ����__________________��
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��_________���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ���� ��
a������ʱ��70�濪ʼ�ռ���Ʒ
b��������ʵ����������
c���Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������_________��
a�������Ը��������Һ b���ý����� c���ⶨ�е�
 ���ݼ���ϵ�д�
���ݼ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ũ���� |
| ���� |
| Ũ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
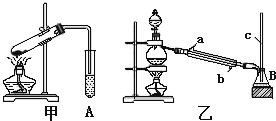
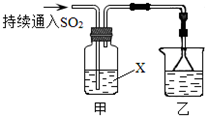 ij��ѧ��ȤС��������װ��̽��SO2��ijЩ��ѧ���ʣ�
ij��ѧ��ȤС��������װ��̽��SO2��ijЩ��ѧ���ʣ� Ca2++
Ca2++ ClO-+
ClO-+ SO2+
SO2+ H2O=
H2O=
 Cl-+
Cl-+ SO42-+
SO42-+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�긣��ʡ�����и���1�µ���������鿼�Ի�ѧ�Ծ��������棩 ���ͣ������
ij��ѧ��ȤС��������װ��̽��SO2��ijЩ��ѧ���ʡ�
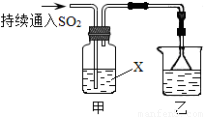
��1��װ���ҵ�������????????????????????????? ��
��2����XΪƷ����Һ���۲쵽��Һ��ɫ��˵��SO2����??????????????? ������ţ���ͬ������XΪNa2S��Һ���۲쵽��Һ�г��ֵ���ɫ���ǣ�˵��SO2����????????????? ��
a��������???????????? b����ԭ��???????????? c��Ư����
��3�����Լ�XΪCa(ClO)2��Һ���ɹ۲쵽��ɫ�������ɣ�����ù��̵����ӷ���ʽ��
 Ca2��+
Ca2��+ ClO��+
ClO��+ SO2+
SO2+ H2O��
H2O�� ????? ��+
????? ��+ Cl��+
Cl��+ SO42��+
SO42��+ ???? ��
???? ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012���㽭������ѧ�߶���ѧ�ڵڶ����¿���ѧ����7��8�ࣩ�������棩 ���ͣ�ʵ����
ij��ѧ��ȤС��������װ��,�Ի������Ʊ�����ϩ

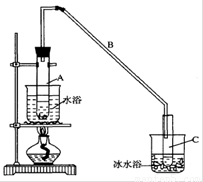
(1)�Ʊ���Ʒ
��12.5mL�����������Թ�A�У��ټ���lmLŨ���ᣬҡ�Ⱥ�������Ƭ����������
����Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
�ٵ���B���˵�������е�������____________��
���Թ�C���ڱ�ˮԡ�е�Ŀ����________________��
��2)�Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��_________��(���ϻ���)����Һ����_________ (������)ϴ�ӡ�
a��KMnO4��Һ b��ϡH2SO4 c��Na2CO3��Һ
���ٽ�����ϩ��ͼװ��������ȴˮ ��_________�ڽ��롣����ʱҪ������ʯ�ң�Ŀ����__________________��
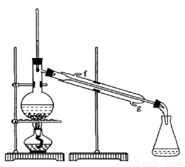
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��_________���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ���� ��
a������ʱ��70�濪ʼ�ռ���Ʒ
b��������ʵ����������
c���Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������_________��
a�������Ը��������Һ b���ý����� c���ⶨ�е�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com