
(1)±ł¶¾µÄĦ¶ūÖŹĮæ£ŗ__________£¬·Ö×ÓŹ½£ŗ__________”£
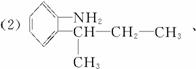
(2)±ł¶¾µÄĶ¬·ÖŅģ¹¹ĢåÓŠŗܶą£¬Š“³öĶ¬Ź±·ūŗĻĻĀĮŠČżĻīŅŖĒóµÄ±ł¶¾µÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½(Ö»ŅŖĒ󊓳öĘäÖŠµÄČżÖÖ)£ŗ____________________”£
¢Ł±½»·ÉĻÖ»ÓŠĮ½øöĻąĮŚµÄČ”“ś»ł£»¢Ś·Ö×ÓÖŠŗ¬ÓŠ°±»ł(”ŖNH2)£»¢Ū·Ö×ÓÖŠŗ¬ÓŠĮ½øö¼×»ł(CH3”Ŗ)
½āĪö£ŗĢ¼ŌŖĖŲŗĶĒāŌŖĖŲŗĻ¼ĘÖŹĮæ·ÖŹżŹĒ90.6%£¬ĖµĆ÷µŖŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ9.4%£¬²ÉÓĆ”°¼«¶Ė¼ŁÉč·Ø”±£¬É豳¶¾µÄĻą¶Ō·Ö×ÓÖŹĮæŹĒ150£¬Ōņ1 mol·Ö×ÓÖŠµŖµÄÖŹĮæĪŖ150”Į9.4%=14.1(g)£¬æÉÖŖŅ»øö±ł¶¾·Ö×ÓÖŠÓŠŅ»øöµŖŌ×Ó”£ŌŁÓĆ14³żŅŌ9.4%£¬ŌņŌ¼µČÓŚ149£¬ÓÖÖŖ·Ö×ÓÖŹĮæ²»³¬¹ż150£¬ŌņČ·¶ØĘäĦ¶ūÖŹĮæŹĒ149 g”¤mol-1”£ÓĆĢÖĀŪµÄ·½·ØČ·¶ØĘä·Ö×ÓŹ½ŹĒC10H15N”£
“š°ø£ŗ(1)149 g”¤mol-1 C10H15N
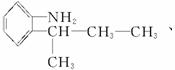
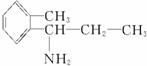
ŅŌ¼°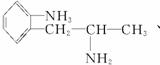
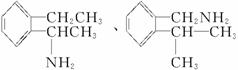 µČ”£
µČ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com