
| Fe��OH��2 | Fe�� OH��3 | Cu��OH��2 | Zn��OH��2 | Mn��OH��2 | |
| ��ʼ������pH | 7.5 | 2.2 | 5.2 | 6.4 | 8.6 |
| ������ȫ��pH | 9.0 | 3.2 | 6.7 | 8.0 | 10.1 |
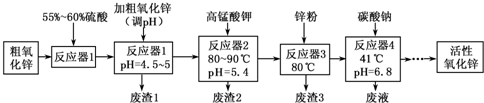
���� ������п����ϡ�����ܽ⣬���ˣ��õ���Һ�к�������п������������������������ͭ�������̣����������Ե�����ҺpH=4.5��5��������ת��ΪFe�� OH��3���������˷��룬�ټ�����������Һ������������Ϊ�����ӣ��ɣ�2���з�Ӧ��2�з�Ӧ���ӷ���ʽ���������ӱ�����MnO2�����˷��룬��Һ�м���Zn�ۣ��û���Cu����ȥ��Һ��ͭ���ӣ����˷��룬��Һ����Ҫ��������п������أ�����̼���ƣ��ɣ�7�����õ���ʽ̼��п�����ȷֽ����ɻ�������п��
��1�����������Ե�����ҺpH=4.5��5��������ת��ΪFe�� OH��3������
��2��MnO4-��MnԪ����+7�۽���ΪMnO2��+4�ۣ�������3�ۣ�Mn2+��+2������ΪMnO2��+4�ۣ�����2�ۣ����ϼ�������С������Ϊ6����MnO4-��ϵ��Ϊ2��Mn2+��ϵ��Ϊ3����ԭ���غ��֪ȱ��Ϊˮ���ٽ��ԭ���غ㡢����غ���ƽ��
��3��պȡ����Ӧ��2���е���Һ���ڵ��۵⻯����ֽ�ϣ�����۲쵽��ֽ������˵��KMnO4������
��4����֪���٣�2MnO2��s��+C��s���T2MnO��s��+CO2��g����H=-174.6kJ•mol-1
�ڣ�C��s��+CO2��g���T2CO��g����H=+283.0kJ•mol-1
���ݸ�˹���ɣ�����-�ڣ���2�ɵã�MnO2��s��+CO��g��=MnO��s��+CO2��g����
��5��������ҺpH����ȥ��Һ��Cu2+��
��6������Ӧ��4���õ��ķ�Һ����Ҫ��������ء������ƣ�
��7���ֽ�������������п��������̼��ˮ�����������غ��������ˮ����������������п��������̼��ˮ�����ʵ������ٸ���ԭ���غ�����ʽ̼��п��ZnCO3��Zn��OH��2��H2O�����ʵ���֮��ȷ����ɣ�
��� �⣺������п����ϡ�����ܽ⣬���ˣ��õ���Һ�к�������п������������������������ͭ�������̣����������Ե�����ҺpH=4.5��5��������ת��ΪFe�� OH��3���������˷��룬�ټ�����������Һ������������Ϊ�����ӣ��ɣ�2���з�Ӧ��2�з�Ӧ���ӷ���ʽ���������ӱ�����MnO2�����˷��룬��Һ�м���Zn�ۣ��û���Cu����ȥ��Һ��ͭ���ӣ����˷��룬��Һ����Ҫ��������п������أ�����̼���ƣ��ɣ�7�����õ���ʽ̼��п�����ȷֽ����ɻ�������п��
��1�����������Ե�����ҺpH=4.5��5��������ת��ΪFe�� OH��3������
�ʴ�Ϊ��Fe�� OH��3��
��2��MnO4-��MnԪ����+7�۽���ΪMnO2��+4�ۣ�������3�ۣ�Mn2+��+2������ΪMnO2��+4�ۣ�����2�ۣ����ϼ�������С������Ϊ6����MnO4-��ϵ��Ϊ2��Mn2+��ϵ��Ϊ3����ԭ���غ��֪ȱ��Ϊˮ���ٽ��ԭ���غ㡢����غ���ƽ����Ӧ���ӷ���ʽΪ��2MnO4-+3Mn2++2H2O=5MnO2��+4H+��
�ʴ�Ϊ��2��3��2H2O��5��4
��3��պȡ����Ӧ��2���е���Һ���ڵ��۵⻯����ֽ�ϣ�����۲쵽��ֽ������˵��KMnO4������
�ʴ�Ϊ�����۵⻯�أ�
��4����֪���٣�2MnO2��s��+C��s���T2MnO��s��+CO2��g����H=-174.6kJ•mol-1
�ڣ�C��s��+CO2��g���T2CO��g����H=+283.0kJ•mol-1
���ݸ�˹���ɣ�����-�ڣ���2�ɵã�MnO2��s��+CO��g��=MnO��s��+CO2��g����H=-228.8kJ/mol��
�ʴ�Ϊ��MnO2��s��+CO��g��=MnO��s��+CO2��g����H=-228.8kJ/mol��
��5������Ӧ��3���м���п�۵������ǣ�������ҺpH����ȥ��Һ��Cu2+��
�ʴ�Ϊ��������ҺpH����ȥ��Һ��Cu2+��
��6������Ӧ��4���õ��ķ�Һ����Ҫ��������ء������ƣ����е���Ҫ���ӳ���Na+�⣬����K+��SO42-��
�ʴ�Ϊ��K+��SO42-��
��7���ֽ�������������п��������̼��ˮ���õ�ZnO 2.43g�����ʵ���Ϊ$\frac{2.43g}{81g/mol}$=0.03mol��CO2�����ʵ���Ϊ$\frac{0.224L}{22.4L/mol}$=0.01mol������Ϊ0.01mol��44g/mol=0.44g����ˮ������Ϊ3.41g-2.43g-0.44g=0.54g��ˮ�����ʵ���Ϊ$\frac{0.54g}{18g/mol}$=0.03mol��
��n��ZnCO3��=0.01mol��n[Zn��OH��2]=0.03mol-0.01mol=0.02mol��n��H2O��=0.03mol-0.02mol=0.01mol��
��n��ZnCO3����n[Zn��OH��2]��n��H2O��=0.01mol��0.02mol��0.01mol=1��2��1��
�ʼ�ʽ̼��п�����ΪZnCO3•2Zn��OH��2•H2O��
�ʴ�Ϊ��ZnCO3•2Zn��OH��2•H2O��
���� �����Թ�������Ϊ���壬����ѧ���Ķ���ȡ��Ϣ�������Բ�������ķ������ۡ����ʵķ����ᴿ���Ȼ�ѧ����ʽ��д��������ԭ��Ӧ��ƽ��������ɵIJⶨ�ȣ��Ƕ���ѧ֪ʶ���ۺ������������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ����Ϣ���н�������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Fe2O3��Al2O3��������Ϊ�ܽ⡢���ˡ����� | |
| B�� | ���ο�ͨ���ܽ⡢���ˡ��������ᾧ�ķ����ᴿ | |
| C�� | �����Ȼ�����Һ���ǽ�FeCl3��������ˮ���ټ���һ���������� | |
| D�� | ȡ�ý����ƻ��ʱ��û������ƻ��Ҫ�Ż�ԭƿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Na2CO3��Һ�м����Ũ�ȵ���������c��Na+��=c��CO32-��+c��HCO3-��+c��H2CO3�� | |
| B�� | NaHS��Һ�У�c��OH-��+c��S2-��=c��H+��+c��H2S�� | |
| C�� | pH��ͬ�Ģ�CH3COONa��Һ����NaClO��Һ��c��Na+�����٣��� | |
| D�� | pH=9��0.1mol•L-1��NaHR��Һ�У�c��HR-����c��H+����c��R2-����c��H2R�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
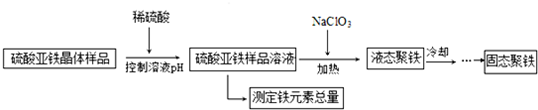
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
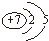 ��
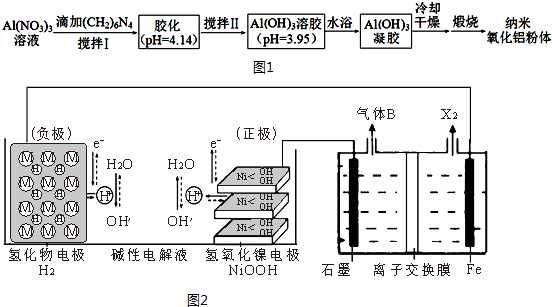
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
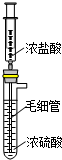 ʵ��������ͼװ���Ʊ�HCl���壮
ʵ��������ͼװ���Ʊ�HCl���壮
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | AgCl�������ɺ��ܽ�ͬʱ�ڲ��Ͻ��У���������� | |
| B�� | AgCl������ˮ������Һ��û��Ag+��Cl- | |
| C�� | ֻҪ����AgCl�ı�����Һ�м������ᣬһ�����г������� | |
| D�� | ����AgCl������Һ�м���NaBr���壬AgCl�������仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CO32- | B�� | HCO3- | C�� | Ba2+ | D�� | NH4+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
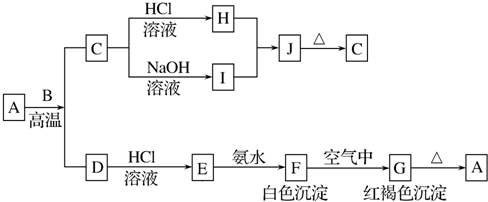
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com