
ij����С���һ����̼��ԭ��������ʵ�������IJ������Ũ�����Ȥ����ͨ��ʵ����̽����ɷ֡�
��ʵ��װ�ã�
��һ����̼��ԭ��������ʵ��װ��
��װ��B�з��������ӷ���ʽ��
װ��B��������
��ʵ����������A�еķ�ĩ�ɺ�ɫ��Ϊ��ɫʱ��ֹͣ���ȣ�����ͨһ����̼����ȴ�����£�ֹͣͨ����ͬʱ�۲쵽�����ʯ��ˮ����ǡ�
��ʵ����ۣ�
����Ϊ����������ʵ����������жϳ����ɵĺ�ɫ����Ϊ��������
����Ϊ����������ʵ����������֤�����ɵĺ�ɫ����Ϊ����������������һ��ʵ�飺�ô����������ɵĺ�ɫ���壬�����к�ɫ���屻�������������ǵó����ɵĺ�ɫ����Ϊ�������Ľ��ۡ�
����ͨ���÷�Ӧ��������϶����ǽ��������жϲ�ͨ��ʵ�����������ԣ�
��1����һ�������£�һ����̼���������ڼ��������£��ɷ������·�Ӧ
3Fe2O3+CO 2Fe3O4+CO2
2Fe3O4+CO2
Fe3O4+4CO 4Fe+4CO2
4Fe+4CO2
��2��������������Fe3O4��Ϊ��ɫ���壬��ǿ���ԣ��ܹ�������������
�ס���ͬѧ�Ľ��ۣ� ��Դ����۵������ǣ�
����ʵ��̽��
�Է�Ӧ�����ɷ�������裺
����1����Ӧ�������ֻ��Fe��
����2����Ӧ�������ֻ��Fe3O4��
����3����Ӧ�������_______________________
Ϊȷ��ʵ�������IJ���ijɷ֣���ͬѧ�������ʵ�飬����������ѡ�Լ���������������ɸ�̽�����̣�������д�ڴ����Ӧλ�á�
��ѡ�Լ��������� 1mol/LCuSO4 ��0.01mol/L KSCN��Һ��1mol/L���ᡢ0.01mol/L��ˮ���Թܡ�����������ͷ�ιܡ�
| ʵ����� | Ԥ������ͽ��� |
| ����һ��ȡӲ�ʲ������й�����������ֱ���A��B�Թ��У���������1mol/LCuSO4��Һ�������ܽ⡣ | ��1����A�Թ��к�ɫ���岻�ܽ⣬����û�й۲쵽�����������ɫ����Ϊ ��2����B�Թ����к�ɫ������������˵����ɫ�����к���Fe�� |
| ����������Թ�B����Һ���ˣ������ù���ϴ�Ӹɾ�������1mol/L����������ηֱ��������0.01mol/L��ˮ������0.01mol/L KSCN��Һ | ��1������Һ�����ɫ���� ��2������Һ���ɫ���� |
��16�֣�
��. Ca2++2OH-+ CO2= CaCO3��+H2O ��2�֣�
����CO�е�CO2������COȼ�ձ���ȥ����2�֣�
��.�ס���ͬѧ�Ľ��۾�����ȷ�������������������Ǻ�ɫ�Ҿ��ܱ�������������1+2�� ��3�֣�
��.����3����Ӧ��������Ⱥ���Fe�ֺ���Fe3O4��1�֣�ʵ����� Ԥ������ͽ��� ��1����A�Թ��к�ɫ���岻�ܽ⣬����û�й۲쵽�����������ɫ����ΪFe3O4��1�֣� ��1������Һ�����ɫ��������1��ȷ ��2�֣�
��2������Һ���ɫ��������3��ȷ��2�֣�
��.�У�1�֣���
��������������ȫ��Ӧ�������������������������������������������������ı仯����ͬ����2�֣�
�������������
��.�����Ŀ��Ϣ����Ӧ����������CO��CO2����֪ʯ��ˮ������������CO2������COȼ�ձ���ȥ��
��.(2) ����Ŀ��Ϣ��������������Fe3O4��Ϊ��ɫ���壬��ǿ���ԣ��ܹ����������������Եõ��𰸡�
��.����һ�У������ֺ�ɫ˵�������û���ͭ���ʣ���û�����ɫ����ΪFe3O4��
������У�Fe������ֻ������Fe2+��Fe3O4������������Fe2+�� Fe3+��
��.��������ȫ��Ӧ�������������������������������ı仯����ͬ��
���㣺������̽��ʵ��Ϊ������������Ԫ�ؼ����������ʡ�̽��������������������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
I����ͼ��ʾ�ӹ��������з���Q��2�ַ�������ش��й����⡣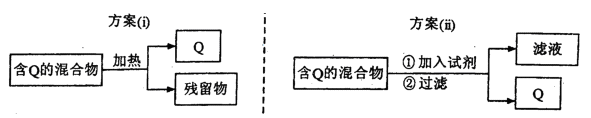
��1��ѡ�÷���(i)ʱ��QӦ�þ��е�������_____________��������Ӧ�þ�
�������__________________________________��
��2��ѡ�÷���(ii)��ij������ĩ������Au��Ag��Cu���з���Au��������Լ�Ϊ____________��
��3��Ϊ�ᴿijFe2O3��Ʒ����Ҫ������SiO2.Al2O3�������շ���(i)��(ii)�������һ���Կ�ͼ��ʽ��ʾ��ʵ�鷽����ע�����ʺͲ�������
______________________________________________________________________________��
��ij�ֺ������������ƵĹ���������Ʒ����֪��Ʒ����Ϊ1.560g����ƿ��ˮ������Ϊ
190.720g)����������ͼ��ʾװ�òⶨ�������Na2O2������������ÿ����ͬʱ����õ�
����ƽ���������±���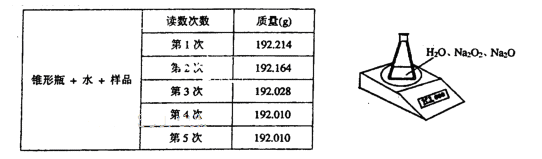
��4��д��Na2O2��H2O��Ӧ�Ļ�ѧ����ʽ��________________________________________��
��5������Na2O2��������ʱ�������������_________________________________________.
��������6�ζ�����ԭ����_____________________________________________________��
��6���ⶨ������Ʒ(1.560g)��Na2O2������������һ�ַ�����������������£�
�����ڵ�������____________���÷�����ֱ�Ӳⶨ����������_____________ ���ⶨ������
��Ҫ�������е�����ƽ�������ƾ��ƣ�����Ҫ___________��__________���̶�����
���������⣩,��ת����Һʱ������Һת�Ʋ���ȫ����Na2O2���������IJⶨ���_______����
��ƫ����ƫС�����䡱����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�ִ�п��Ʒ�ӹ���ҵ���յķ���������ZnO��FeO��Fe2O3��CuO��Al2O3�����ʣ�����ȡ����п���������£�
�й�����������ȫ������pH���±���
| ������ | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 | Zn(OH)2 |
| pH | 5.2 | 3.2 | 9.7 | 6.7 | 8.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
FeSO4��7H2O�׳��̷����㷺����ҽҩ��������
��1����֪FeSO4��7H2O�����ڼ��������·������·�Ӧ��
 ��������ͼװ�ü���÷�Ӧ��������
��������ͼװ�ü���÷�Ӧ��������
����д���пհף�
������������˳��Ϊ ����a��i����ĸ��ʾ����
��װ��C�е��Լ�X��ѧʽΪ ����װ������ˮ�������� ��
��2��������FeSO4��7H2O��ʵ�����Ʊ�����ͼ��
�����������������գ�
�ٷ�Ӧ����Ҫl00mL l��5mol��L-1l��ϡ�����ܽ�ྻ����м������������Ϊ98%���ܶ�Ϊ1.84g��cm-3��Ũ�������ơ����õ���������Ͳ���ձ�������������ͷ�ιܼ� ����ȡŨ��������Ϊ ��
�ڷ�Ӧ1��Ҫ���������ӣ���ԭ���� ������AΪ ��
�۲ⶨFeSO4��7H2O��Ʒ��Fe2+�����ij��÷�����KMnO4��Һ�ζ�������֪��ȡ3.0g FeSO4��7H2O��Ʒ�����Ƴ���Һ���������ữ��0.01000moL��L-1 KMnO4��Һ�ζ�������KMnO4��Һ�����Ϊ200.00mL��������Ӧ�����ӷ���ʽΪ ������������Ʒ��FeSO4��7H2O����������Ϊ ��������λ��Ч���֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������ȣ�ClO2����Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч�����Ĺ�������������һ�ֻ���ɫ�����壬������ˮ��ʵ���ҿ���NH4Cl�����ᡢNaClO2���������ƣ�Ϊԭ�����Ʊ�ClO2�����������£�
��1��д�����ʱ������Ӧ�Ļ�ѧ����ʽ�� ��
��2����ȥClO2�е�NH3��ѡ�õ��Լ��� ��������ĸ��
| A������ʳ��ˮ | B����ʯ�� | C��Ũ���� | D��ˮ |
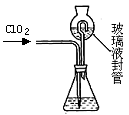
| ʵ�鲽�� | ʵ������ | ʵ����� |
| �� | | ��ҺX�к���Na+ |
| �� | | ��ҺX�к���Cl�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ʵ��װ��ͼ����Щͼ�в��ּг�����δ���������ܴﵽ��ʵ��Ŀ�ĵ���
 |  |
| A��֤�����ԣ����̼����� | B��ʵ������ȡ�������� |
 | 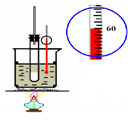 |
| C��ʯ�ͷ��� | D��ʵ������ȡ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ʵ���������
 |  |  |  |
| A��֤���ǽ��� Cl��C��Si | B�����հ����� ����ֹ���� | C���Ʊ����ռ�����NO2���� | D���Ʊ�CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����±��ṩ��������ҩƷ�����ܴﵽ��Ӧʵ��Ŀ�ĵ���
| ��� | ���� | ҩƷ | ʵ��Ŀ�� |
| A | ������ƽ�������룩��250mL����ƿ����Ͳ���ձ���ҩ�ס������� | NaOH���塢����ˮ | ����250mLһ�����ʵ���Ũ�ȵ�NaOH��Һ |
| B | ��Һ©������ƿ�����ܼ���Ƥ�� | ϡ���ᡢ̼���ơ���������Һ | ֤���ǽ����ԣ�S��C��Si |
| C | ��ʽ�ζ��ܡ���ʽ�ζ��ܡ���ͷ�ιܡ�����̨�������У�����ƿ | ��֪Ũ�ȵ�NaOH��Һ���������ᡢ����ˮ����ֽ | �ⶨϡ��������ʵ���Ũ�� |
| D | ����̨�������У����ƾ��ơ����Թܡ�����ƿ�����ܼ���Ƥ�� | �Ȼ�� | ��ȡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����й�ʵ�������Ԥ���װ�õ�ѡ����ȷ����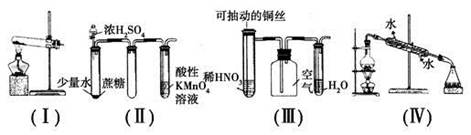
| A����װ��(��)���Ȳ��ᾧ���ȡijЩ����(���ᾧ����۵�101.5 �棬�ֽ��¶�ԼΪ150 ��) |
| B����װ��(��)����ʵ��ʱ������KMnO4��Һ�г������ݣ�����ɫ����ȥ |
| C����װ��(��)����ʵ��ʱ�����ƿ������dz����ɫ������ֺ��ֱ�Ϊ��ɫ���Ҳ������������Ⱦ |
| D����װ��(��)�����屽�ͱ��Ļ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com