
ij�������к�������[KAl(SO4)2]��Al2O3��Fe2O3����һ���������ɼ�ʵ����ͼ��ʾ������֮���ת����
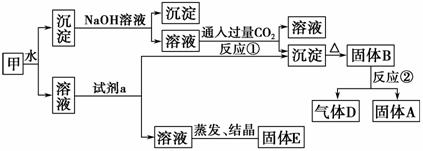
��ش��������⣺
(1)д��A��B��D�Ļ�ѧʽ��A________��B________��D________��
(2)�Լ�a���ѡ��________(ѡ��A��B��C��D)��
A��NaOH��Һ B��ϡ����
C��������̼ D����ˮ
(3)д����Ӧ�ٵ����ӷ���ʽ�� _________________________________��
(4)����E������Ϊ�����ԵĻ�ѧ���ϣ�E���������ʵĻ�ѧʽΪ______________ ______________��
______________��
������������֪��Ϣ�мijɷ֣������ƶ�BΪ����������˷�Ӧ����ͨ�������õ�D(����)��A(������)������ˮ�õ�����ҺΪ������Һ����Һ�к���Al3����K����SO42��������ˮ�õ��ij���Ϊ���������������Ļ����ټ����������ƣ����������ܽ⣬��ͨ�������̼���Եõ����������������Լ�aӦ��Ϊ��ˮ�����������Լ�a������������Һ�������������ð��գ��ɴ˿���֪EΪ����ء�����淋Ļ���
�𰸡�(1)Al��Al2O3��O2��(2)D
(3)[Al(OH)4]����CO2===Al(OH)3����HCO3��
(4)K2SO4��(NH4)2SO4



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±��г��ˢ١���ʮ��Ԫ�������ڱ��е�λ�ã�
| �������� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | �� | �� | |
| 4 | �� |
�������١���ʮ��Ԫ���У���Ԫ�ط�����գ���
�����л������ҪԪ��������������������������ʯ���������Ļ���Ԫ����������������������
��ѧ��������õ�Ԫ��������������������̬�⻯���ˮ��Һ�ʼ��Ե�Ԫ��������������������
�������١���Ԫ�ص�����������Ӧ��ˮ�����У�
������ǿ�����ʵĵ���ʽΪ��������������������������������������
������ǿ�����ʵĻ�ѧʽΪ��������������������������������������
������Ԫ�آܺ͢ݵĵ���Ϊ�缫����Ԫ�آ۵�����������Ӧ��ˮ�����ˮ��Һ���ԭ��أ���ܵĵ����ڴ�ԭ��������������������������������
��Ԫ�آߵ�ij������Ϊ�д̼�����ζ����ɫ���壬���⻯��Ϊ�г�������ζ����ɫ���塣�������������ϣ�������һ�ֵ���ɫ��ĩ���˷�Ӧ��ѧ����ʽΪ�������������������������������������������������˷�Ӧ���������������Ϊ3.2g����Ӧ��ת�Ƶĵ�����Ϊ����������������������������ֵ����
��Ԫ�آ��Ԫ�آ����ߺ˵����֮����������������������Ԫ���зǽ����Խ�����Ԫ������������������Ԫ�����ƣ�����˵��������Ԫ�صķǽ�����ǿ����ʵ����ʵ�ǣ������ӷ���ʽ��ʾ��
����������������������������������������������������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������a��b����п���У��ֱ���������ҵ�����ϡH2SO4��ͬʱ��a�м���������CuSO4��Һ����ͼ��ʾ����H2�������V����ʱ�䣨t���Ĺ�ϵ��������ȷ���ǣ� ��
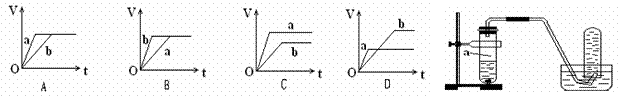

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
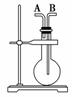 �����������壺H2��Cl2��CH4��HCl��NH3��NO��H2S��SO2���� �� ͼװ�ý���ʵ�飬��д���пհף�
�����������壺H2��Cl2��CH4��HCl��NH3��NO��H2S��SO2���� �� ͼװ�ý���ʵ�飬��д���пհף�
(1)����ƿ����ʱ����A�ڽ������ռ��������� ________________����B�ڽ������ռ���������________��
(2)����ƿ�г���ˮʱ������������________������������
(3)����ƿ��װ��ij����Һ������ϴ��ʱ������Ӧ��____ ____�ڽ�����ƿ��
____�ڽ�����ƿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�������У����������Ȳ���������Ȼ��������ܽ⡱������Ǣ���̼������Һ��ͨ�������CO2������Na[Al(OH)4]��Һ����μ��������ϡ�������AlCl3��Һ����μ��������ϡ����������Һ�������������Һ����μ������������ (����)��
A���٢� B���٢� C���٢� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ͻ�ȴ������ƳɵĽ������ϵ��ŵ��� (����)��
�ٺϽ��Ӳ��һ������ĸ��ɷֽ����Ĵ�һ��أ��Ͻ���۵�����ĸ��ɷֽ����ĸ��͡��۸ı�ԭ�ϵ���ȡ��ı����ɺϽ���������õ��в�ͬ���ܵĺϽ𡡢ܺϽ�ȴ������ĵ����Ը�ǿ���ݺϽ�ȴ�������Ӧ�÷�Χ���㷺
A���٢ڢۢ� B���ڢۢ�
C���٢ڢ� D���٢ڢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Ų��Ͽ�ѧ�ķ�չ�����������仯����õ���Խ��Խ�㷺��Ӧ�ã�������Ϊ���Ͻ��ά���ء���Ϊ�������ú�������(����V2O5��VOSO4�������Բ���)��������Ա����������һ�����ӽ��������շ����¹��գ������ʴ�91.7%���ϡ����ֺ���������ˮ�е��ܽ��������ʾ��
| ���� | VOSO4 | V2O5 | NH4VO3 | (VO2)2SO4 |
| �ܽ��� | ���� | ���� | ���� | ���� |
�ù��յ���Ҫ�������¡�
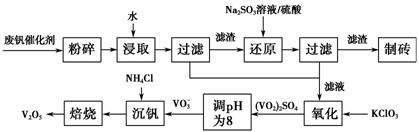
��ش��������⣺
(1)��д������Na2SO3��Һ������Ӧ�����ӷ���ʽ__________________��
(2)��������ʹ�õĴ�������ý(V2O5)�ܼӿ����������������ʣ��˹����в�����һ�������м���(��ͼ1)������a��c�����Ļ�ѧ����ʽ�ɱ�ʾΪ________________________��________________________________��
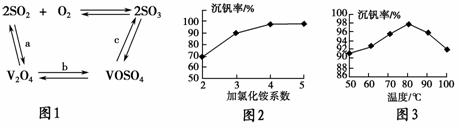
(3)�ù����г������ǻ��շ��Ĺؼ�֮һ�������ʵĸߵͳ�����ҺpHӰ���⣬����Ҫ�����Ȼ�淋�ϵ��(NH4Cl������������Һ��V2O5��������)���¶ȡ�����ͼ2��ͼ3���Խ�������Ȼ��ϵ�����¶ȵ�����ֵ�ֱ�Ϊ________��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����
A��1molO��������32g B��HCl��Է�������36.5g/mol
C��SO42-��Ħ��������96g/mol D��Na�����ԭ������23g/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ÿ���и������ڼ������Ӽ����й��ۼ���һ���� (����)��
A��NaOH��H2SO4��(NH4)2SO4
B��MgO��Na2SO4��NH4HCO3
C��Na2O2��KOH��Na2SO4
D��HCl��Al2O3��MgCl2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com