
ij�о���ѧϰС�������ͼװ����ȡ������������Ϊԭ�Ͻ���ʵ�顣
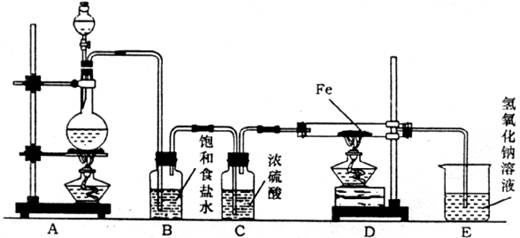
��1��װ��A��ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��װ��B�б���ʳ��ˮ�������� ��װ��C��Ũ����������� ��
��3��ʵ��ʱ���ȵ�ȼ ���ľƾ��ƣ��ٵ�ȼ ���ƾ��ƣ�д��D�з�Ӧ�Ļ�ѧ����ʽ ��д��E�з�Ӧ�����ӷ���ʽ ��
��4����ʵ������12mol?L��1Ũ����10mL��������MnO2��Ӧ������Cl2�����ʵ�������С��0.03mol���Է������ܴ��ڵ�ԭ���Ǣ� ���� ����ʹ��Ӧ����Cl2�����ʵ������̶ȵĽӽ�0.03mol������װ�����������õ�ǰ����ʵ����Ӧ��ȡ�Ĵ�ʩ�� ��
 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

- 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
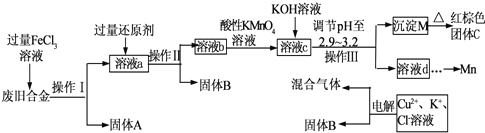
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
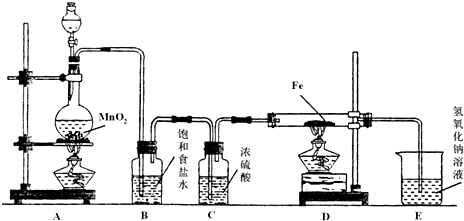
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
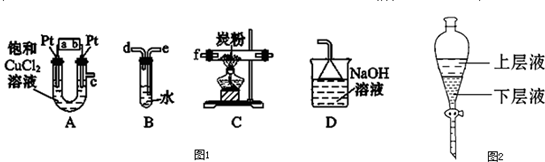
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
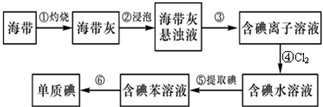
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com