
| ČÜÖŹ | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN |
| pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 |
·ÖĪö £Ø1£©ČõĖįøłÖŹĮæĖ®½ā³Ģ¶ČŌ½“ó£¬ĻąĶ¬ÅØ¶ČµÄÄĘŃĪČÜŅŗµÄpHŌ½“ó£¬ŌņĖįøłĄė×Ó½įŗĻÖŹ×ÓÄÜĮ¦Ō½“ó£»
£Ø2£©ĒæĖįÄÜŗĶČõĖįŃĪ·“Ӧɜ³ÉøüČõµÄĖį£»
£Ø3£©ĖįŠŌHCl£¾H2CO3£¾HClO£¾HCO3-£¬ĀČĖ®ÖŠHClÄÜŗĶĢ¼ĖįÄĘ”¢Ģ¼ĖįĒāÄʶ¼·“Ó¦£¬HClOÄÜŗĶĢ¼ĖįÄĘ·“Ó¦µ«ŗĶĢ¼ĖįĒāÄĘ²»·“Ó¦£»
£Ø4£©Ģ¼ĖįÄĘŹĒĒæ¼īČõĖįŃĪ£¬Ģ¼ĖįøłĄė×ÓĮ½²½Ė®½āÉś³ÉĒāŃõøłĄė×Ó¶ųµ¼ÖĀĘäĖ®ČÜŅŗ³Ź¼īŠŌ£¬Ę䵌Ņ»²½Ė®½ā³Ģ¶ČŌ¶Ō¶“óÓŚµŚ¶ž²½£¬ŌŁ½įŗĻµēŗÉŹŲŗćÅŠ¶Ļ£»
£Ø5£©Ėį»ņ¼īŅÖÖĘĖ®µēĄė£¬ŗ¬ÓŠČõĄė×ÓµÄŃĪ“Ł½ųĖ®µēĄė£¬pH=11µÄNaOHŗĶNaCNČÜŅŗÖŠ£¬ÓÉĖ®µēĄė³öµÄc£ØH+£©·Ö±šĪŖ10-11 mol/L”¢10-3 mol/L£»
£Ø6£©³£ĪĀĻĀ£¬½«2amol/LµÄHCNČÜŅŗÓėamol/LµÄNaOHČÜŅŗµČĢå»ż»ģŗĻ£¬ČÜŅŗÖŠČÜÖŹĪŖµČĪļÖŹµÄĮæÅØ¶ČµÄHCNŗĶNaCN£¬²āµĆ»ģŗĻŅŗµÄpH=8£¬ČÜŅŗÖŠ“ęŌŚµēŗÉŹŲŗćŗĶĪļĮĻŹŲŗć£¬øł¾ŻµēŗÉŹŲŗćŗĶĪļĮĻŹŲŗćÅŠ¶Ļ£®
½ā“š ½ā£ŗ£Ø1£©ČõĖįøłÖŹĮæĖ®½ā³Ģ¶ČŌ½“ó£¬ĻąĶ¬ÅØ¶ČµÄÄĘŃĪČÜŅŗµÄpHŌ½“ó£¬ŌņĖįøłĄė×Ó½įŗĻÖŹ×ÓÄÜĮ¦Ō½“ó£¬øł¾ŻČÜŅŗpHÖŖ£¬CO32-µÄĖ®½ā³Ģ¶Č×ī“ó£¬ŌņCO32-½įŗĻÖŹ×ÓÄÜĮ¦×īĒ棬¹Ź“š°øĪŖ£ŗCO32-£»
£Ø2£©ĖįµÄĖįŠŌŌ½Ē棬ĘäĖįøłĄė×ÓĖ®½ā³Ģ¶ČŌ½Čõ£¬ĻąĶ¬ÅØ¶ČµÄÄĘŃĪĘäČÜŅŗµÄpHŌ½Š”£¬øł¾ŻÄĘŃĪČÜŅŗpH“óŠ”Ė³ŠņÖŖ£¬ĖįµÄĒæČõĖ³ŠņŹĒCH3COOH£¾H2CO3£¾HClO£¾HCN£¾HCO3-£¬
øł¾ŻĒæĖįÖĘČ”ČõĖįÖŖ£¬Ö»ÓŠD²»·ūŗĻ£¬ĖłŅŌD“ķĪó£¬
¹Ź“š°øĪŖ£ŗD£»
£Ø3£©ĖįŠŌHCl£¾H2CO3£¾HClO£¾HCO3-£¬ĀČĖ®ÖŠHClÄÜŗĶĢ¼ĖįÄĘ”¢Ģ¼ĖįĒāÄʶ¼·“Ó¦£¬HClOÄÜŗĶĢ¼ĖįÄĘ·“Ó¦µ«ŗĶĢ¼ĖįĒāÄĘ²»·“Ó¦£¬ĖłŅŌæÉŅŌÓĆĢ¼ĖįÄĘČÜŅŗŹµĻÖ£¬Ąė×Ó·“Ó¦·½³ĢŹ½ĪŖCl2+HCO3-=Cl-+CO2”ü+HClO£¬
¹Ź“š°øĪŖ£ŗNaHCO3£»Cl2+HCO3-=Cl-+CO2”ü+HClO£»
£Ø4£©Ģ¼ĖįÄĘŹĒĒæ¼īČõĖįŃĪ£¬Ģ¼ĖįøłĄė×ÓĮ½²½Ė®½āÉś³ÉĒāŃõøłĄė×Ó¶ųµ¼ÖĀĘäĖ®ČÜŅŗ³Ź¼īŠŌ£¬Ė®½āĄė×Ó·½³ĢŹ½ĪŖCO32-+H2O?HCO3-+OH-£¬Ę䵌Ņ»²½Ė®½ā³Ģ¶ČŌ¶Ō¶“óÓŚµŚ¶ž²½£¬ŌŁ½įŗĻµēŗÉŹŲŗćµĆc£ØNa+£©£¾c£ØCO32-£©£¬ČÜŅŗÖŠĄė×ÓÅØ¶Č“óŠ”Ė³ŠņŹĒc£ØNa+£©£¾c£ØCO32-£©£¾c£ØOH-£©£¾c£ØHCO3-£©£¾c£ØH+£©£¬
¹Ź“š°øĪŖ£ŗCO32-+H2O?HCO3-+OH-£»c£ØNa+£©£¾c£ØCO32-£©£¾c£ØOH-£©£¾c£ØHCO3-£©£¾c£ØH+£©£»
£Ø5£©Ėį»ņ¼īŅÖÖĘĖ®µēĄė£¬ŗ¬ÓŠČõĄė×ÓµÄŃĪ“Ł½ųĖ®µēĄė£¬pH=11µÄNaOHŗĶNaCNČÜŅŗÖŠ£¬ÓÉĖ®µēĄė³öµÄc£ØH+£©·Ö±šĪŖ10-11 mol/L”¢10-3 mol/L£¬ŌņĮ½ÖÖČÜŅŗÖŠĖ®µēĄė³öµÄĒāĄė×ÓÅضČÖ®±Č=10-11 mol/L£ŗ10-3 mol/L=10-8£ŗ1£¬¹Ź“š°øĪŖ£ŗ10-8£ŗ1£»
£Ø6£©³£ĪĀĻĀ£¬½«2amol/LµÄHCNČÜŅŗÓėamol/LµÄNaOHČÜŅŗµČĢå»ż»ģŗĻ£¬ČÜŅŗÖŠČÜÖŹĪŖµČĪļÖŹµÄĮæÅØ¶ČµÄHCNŗĶNaCN£¬²āµĆ»ģŗĻŅŗµÄpH=8£¬ČÜŅŗÖŠ“ęŌŚµēŗÉŹŲŗćŗĶĪļĮĻŹŲŗć£¬
øł¾ŻµēŗÉŹŲŗćµĆc£ØNa+£©+c£ØH+£©=c£ØOH-£©+c£ØCN-£©
øł¾ŻĪļĮĻŹŲŗćµĆ2c£ØNa+£©=c£ØHCN£©+c£ØCN-£©
ĖłŅŌc£ØHCN£©-c£ØCN-£©=2[c£ØOH-£©-c£ØH+£©]=2£Ø10-6-10-8£©mol/L£¬
¹Ź“š°øĪŖ£ŗ2£Ø10-6-10-8£©mol/L£®
µćĘĄ ±¾Ģāæ¼²éĖį¼ī»ģŗĻČÜŅŗ¶ØŠŌÅŠ¶Ļ”¢ŃĪĄąĖ®½ā”¢ĒæĖįÖĘČ”ČõĖįµČÖŖŹ¶µć£¬ĪŖøßĘµæ¼µć£¬²ąÖŲæ¼²éѧɜ·ÖĪö¼ĘĖćÄÜĮ¦£¬Ć÷Č·ĖįµÄµēĄė³Ģ¶ČÓėĘäĖįøłĄė×ÓĖ®½ā³Ģ¶Č¹ŲĻµŹĒ½ā±¾Ģā¹Ų¼ü£¬×¢Ņā£ŗĢ¼ĖįøłĄė×Ó¶ŌÓ¦µÄĖįŹĒĢ¼ĖįĒāøłĄė×Ó¶ų²»ŹĒĢ¼Ėį£¬ĪŖŅדķµć£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
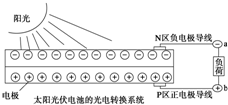
| A£® | ¹ā·ü·¢µēŹĒ½«Ģ«ŃōÄÜ×Ŗ±äĪŖµēÄÜ | |
| B£® | GaÓėNŌŚŌŖĖŲÖÜĘŚ±ķÖŠ²»“¦ÓŚĶ¬Ņ»Ö÷×å | |
| C£® | YAGÖŠīĘĻŌ+3¼Ū | |
| D£® | ÉĻĶ¼ÖŠNĒų°ėµ¼ĢåĪŖøŗ¼«£¬PĒų°ėµ¼ĢåĪŖÕż¼«£¬µēĮ÷“ÓaĮ÷Ļņb |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µ±pH=7Ź±£¬c£ØNa+ £©=c£ØCH3 COO- £©£¾c£ØH+ £©=c£ØOH- £© | |
| B£® | µ±pH£¾7Ź±£¬c£ØCH3 COO- £©£¾c£ØNa+ £©£¾c£ØOH- £©£¾c£ØH+ £© | |
| C£® | µ±Ē”ŗĆĶźČ«ÖŠŗĶŹ±£¬c£ØNa+ £©£¾c£ØCH3 COO- £©£¾c£ØOH- £©£¾c£ØH+ £© | |
| D£® | ĪŽĀŪČÜŅŗĻŌŹ²Ć“ŠŌ¶¼ÓŠ¹ŲĻµ£ŗc£ØNa+ £©+c£ØH+ £©=c£ØCH3 COO- £©+c£ØOH- £© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĆŗÖŠŗ¬ÓŠ±½ŗĶ¼×±½£¬æÉÓĆĻČøÉĮóŗó·ÖĮóµÄ·½·Ø½«ĖüĆĒ·ÖĄė³öĄ“ | |
| B£® | ŗ¬C18ŅŌÉĻĶéĢžµÄÖŲÓĶ¾¹ż“ß»ÆĮŃ»ÆæÉŅŌµĆµ½ĘūÓĶ | |
| C£® | ĆŗŹĒÓÉÓŠ»śĪļŗĶĪŽ»śĪļ×é³ÉµÄø“ŌÓ»ģŗĻĪļ | |
| D£® | ŹÆÓĶŗ¬ÓŠC5”«C11µÄĶéĢž£¬æÉŅŌĶعżŹÆÓĶµÄ·ÖĮóµĆĘūÓĶ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | HCl | B£® | NaOH | C£® | Na 2SO 4 | D£® | NaCl |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
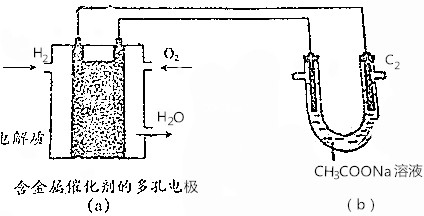
| A£® | Čō£Øa£©³ŲĻūŗÄ2.24LH2£¬Ōņ£Øb£©³Ų£ØC1£©¼«²śÉś0.2mol CO2 | |
| B£® | £Øa£©³ŲČ¼ĮĻµē³ŲÖŠÕż¼«·“Ó¦Ź½ĪŖO2+4e-+4H+ØT2H2O | |
| C£® | ½«£Øb£©³Ųµē½āŗóµÄČÜŅŗ¼ÓČČÕōøÉ£¬µĆµ½NaOH¹ĢĢå | |
| D£® | £Øb£©³ŲÓŅ²ąŹÆÄ«µē¼«£ØC2£©ĪŖµē½ā³ŲŅõ¼« |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | C2H6 | B£® | C3H6 | C£® | C3H8 | D£® | C4H8 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | Va£¾VbŹ±£ŗc £ØCH3COOH£©£¾c £ØCH3COO-£©£¾c £ØK+£© | |
| B£® | Va=VbŹ±£ŗc £ØCH3COOH£©+c £ØH+£©£¾c £ØOH-£© | |
| C£® | Va£¼VbŹ±£ŗc £ØCH3COO-£©£¾c £ØK+£©£¾c £ØOH-£©£¾c £ØH+£© | |
| D£® | VaÓėVbČĪŅā±ČŹ±£ŗc £ØK+£©+c £ØH+£©=c £ØOH-£©+c £ØCH3COO-£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ±ź×¼×“æöĻĀ£¬5.6 L CO2Óė×ćĮæNa2O2·“Ó¦×ŖŅʵĵē×ÓŹżĪŖ0.5 NA | |
| B£® | 78 g±½ŗ¬ÓŠĢ¼Ģ¼Ė«¼üµÄŹżÄæĪŖ3 NA | |
| C£® | ³£ĪĀĻĀ£¬4.4gCO2ŗĶN2O»ģŗĻĪļÖŠĖłŗ¬ÓŠµÄŌ×ÓŹżĪŖ0.3 NA | |
| D£® | 1 L 1 mol•L-1µÄCuSO4ČÜŅŗÖŠŗ¬NAøöCu2+ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com