



 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣���ҵ�����г�������NaCl��Na2SO4�����ʡ�Ϊ�ⶨij��ҵ����Ĵ��ȣ����������ͼʵ��װ�á�
����ʵ����ƣ���ش�
(1��װ����ʢװϡ��������������� ��
װ��D��������
��
(2��Ϊ�ﵽ�ⶨij��ҵ����Ĵ���ʵ��Ŀ�ģ�һ��ʵ��������Ӧ ���г��������Ĵ���Ϊ ��
(3���������Dz�����ҩƷ���������ȷ���
��Ӱ�죬��ʵ���ý���� (�ƫ�ߡ�����ƫ�͡�������Ӱ�족����
ԭ����
(������Ϊ��Ӱ�죬�ÿղ��ûش𣩡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ������ѧ�߶���ѧ����ĩ���Ի�ѧ ���ͣ�ʵ����
��10�֣���ҵ�����г�������NaCl��Na2SO4�����ʡ�Ϊ�ⶨij��ҵ����Ĵ��ȣ����������ͼʵ��װ�á�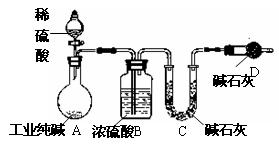
����ʵ����ƣ���ش�
(1��װ����ʢװϡ��������������� ��
װ��D��������
��
(2��Ϊ�ﵽ�ⶨij��ҵ����Ĵ���ʵ��Ŀ�ģ�һ��ʵ��������Ӧ���г��������Ĵ���Ϊ ��
(3���������Dz�����ҩƷ���������ȷ���
��Ӱ�죬��ʵ���ý���� (�ƫ�ߡ�����ƫ�͡�������Ӱ�족����
ԭ����
(������Ϊ��Ӱ�죬�ÿղ��ûش𣩡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�߶���ѧ����ĩ���Ի�ѧ ���ͣ�ʵ����
��10�֣���ҵ�����г�������NaCl��Na2SO4�����ʡ�Ϊ�ⶨij��ҵ����Ĵ��ȣ����������ͼʵ��װ�á�
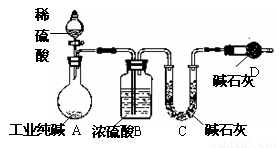
����ʵ����ƣ���ش�
(1��װ����ʢװϡ��������������� ��
װ��D��������
��
(2��Ϊ�ﵽ�ⶨij��ҵ����Ĵ���ʵ��Ŀ�ģ�һ��ʵ��������Ӧ ���г��������Ĵ���Ϊ ��
(3���������Dz�����ҩƷ���������ȷ���
��Ӱ�죬��ʵ���ý���� (�ƫ�ߡ�����ƫ�͡�������Ӱ�족����
ԭ����
(������Ϊ��Ӱ�죬�ÿղ��ûش𣩡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣���ҵ�����г�������NaCl��Na2SO4�����ʡ�Ϊ�ⶨij��ҵ����Ĵ��ȣ����������ͼʵ��װ�á�
����ʵ����ƣ���ش�
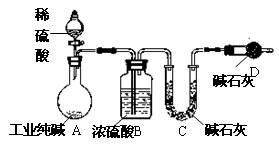 (1��װ����ʢװϡ��������������� ��
(1��װ����ʢװϡ��������������� ��
װ��D��������
��
(2��Ϊ�ﵽ�ⶨij��ҵ����Ĵ���ʵ��Ŀ�ģ�һ��ʵ��������Ӧ ���г��������Ĵ���Ϊ ��
(3���������Dz�����ҩƷ���������ȷ���
��Ӱ�죬��ʵ���ý���� (�ƫ�ߡ�����ƫ�͡�������Ӱ�족����
ԭ����
(������Ϊ��Ӱ�죬�ÿղ��ûش𣩡�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com