
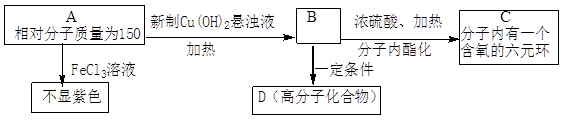
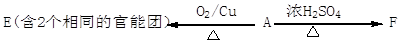
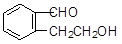
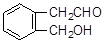
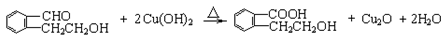
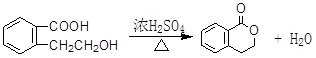
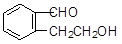
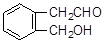
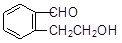
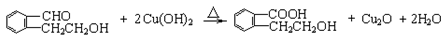 ��
��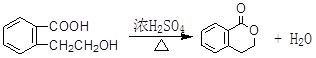 ��
��

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
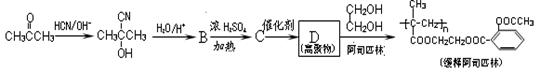
 ���ϳ�·�ߵ�һ���֡����������������¿����ģ�2���ٵľ����ٴ�����ϵ������Ȳ�ڼ״���һ����̼�����£���60�桢6 MPa�������ʻ�����һ���Ƶ�
���ϳ�·�ߵ�һ���֡����������������¿����ģ�2���ٵľ����ٴ�����ϵ������Ȳ�ڼ״���һ����̼�����£���60�桢6 MPa�������ʻ�����һ���Ƶ�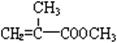 ���仯ѧ����ʽΪ�� ��
���仯ѧ����ʽΪ�� ��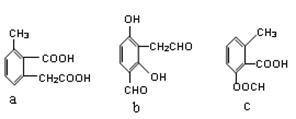
| A��װ����ǰ���ζ���δ�ñ���Һ��ϴ |
| B���ⶨ�������ʱ��ʼ���Ӷ���������Ӷ��� |
| C����ƿ�ñ�����������Һ��ϴ |
| D���ζ����������ὦ��ƿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
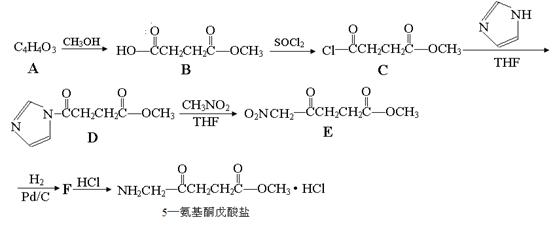
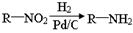
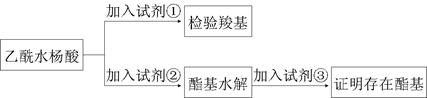
 ����������������Ϣ��д����CH3CH2COOH��
����������������Ϣ��д����CH3CH2COOH�� Ϊԭ�Ϻϳ�
Ϊԭ�Ϻϳ� ����ĺϳ�·������ͼ�����Լ���ѡ����
����ĺϳ�·������ͼ�����Լ���ѡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
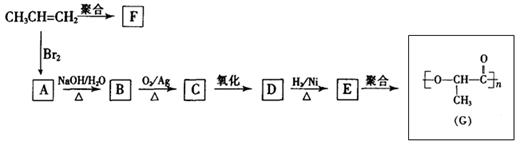
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
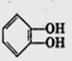
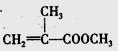 �����л������ĵ��壬������ں˴Ź�������ͼ������ʾ_________����ͬ�ķ塣�����������ڼ���ϩ�����ͬ���칹����� ____ ����ѡ����ţ���CH3COOCH2CH=CH2����CH2=C��CH2CH3��COOCH3����
�����л������ĵ��壬������ں˴Ź�������ͼ������ʾ_________����ͬ�ķ塣�����������ڼ���ϩ�����ͬ���칹����� ____ ����ѡ����ţ���CH3COOCH2CH=CH2����CH2=C��CH2CH3��COOCH3����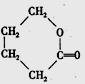 ����
����
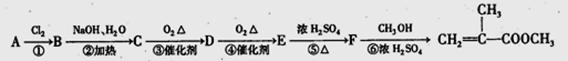
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
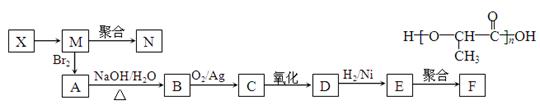
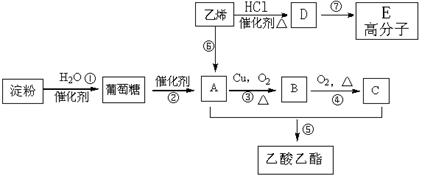
 ���㻯����A��B��Ϊͬ���칹��,B�Ľṹ��ʽ�� CH3COO�� ��COOCH2CH3 ,A���١���������Ӧ��C��D��E��B���١���������Ӧ��E��F��H��������Ӧ���̡��������ʼ����ϵ����ͼ��ʾ:
���㻯����A��B��Ϊͬ���칹��,B�Ľṹ��ʽ�� CH3COO�� ��COOCH2CH3 ,A���١���������Ӧ��C��D��E��B���١���������Ӧ��E��F��H��������Ӧ���̡��������ʼ����ϵ����ͼ��ʾ:
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
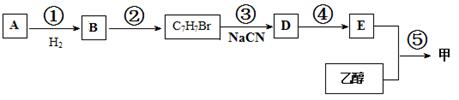
 R��COOH
R��COOH�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com