
ʵ��������ͼ��ʾ��װ����ȡ����������
(1)�ڴ��Թ�������һ���������Ҵ��������Ũ����Ļ��Һ�����������Ϊ ___________ ____________________________________��Ȼ���������Թ�ʹ֮��Ͼ��ȡ�
(2)װ����ͨ�����ĵ���Ҫ���ڱ��͵�Na2CO3��Һ��Һ���϶����ܲ�����Һ�У�Ŀ���Ƿ�ֹ��Һ�ĵ�������ɵ�����ԭ����____________________________________________��
(3)Ũ����������ǣ���__________________����____________________��
(4)ͼ���ұ��Թ����Լ���_______________��
(5)����õ����������ķ�����__________��������Ҫ�IJ���������____________________��
(6)���ӵ���C2H518OHд�������������ķ���ʽ____________________________________��
(7)д��������̼�����Ʒ�Ӧ����ʽ_____________________________________��
��1������Թ���ע�������Ҵ�����ŨH2SO4�����Ҵ��У��ӱ�������������
��2���ӷ������Ҵ�������������ˮ ������ˮ����ѹǿ��С��������2�֣�
��3����������ˮ�� ����1�֣� ��4������̼������Һ��1�֣�
(5) ��Һ��1�֣� ����Һ©�����ձ�����1�֣�
(6) CH3COOH + CH3CH218OH CH3CO18OCH2CH3 +
H2O ��2�֣�
CH3CO18OCH2CH3 +
H2O ��2�֣�
(7) CH3COOH + NaHCO3 ====CH3COONa + CO2 �� + H2O ��2�֣�
��������
�����������1������Ũ��������ˮ���ȣ����Ҵ������ᶼ���ӷ��ģ������ڴ��Թ�������һ���������Ҵ��������Ũ����Ļ��Һ�IJ�������Ϊ����Թ���ע�������Ҵ�����ŨH2SO4�����Ҵ��У��ӱ������������ᡣ
��2�����ڻӷ������Ҵ�������������ˮ������ˮ����ѹǿ��С���Ӷ���������
��3���������Ҵ�����������Ӧ����Ũ�������������÷�ӦΪ���淴Ӧ��Ũ������ˮ����ƽ���������������������ƶ���Ũ���������Ϊ��������ˮ����
��4���Ʊ���������ʱ���ñ���̼������Һ��Ŀ�����кͻӷ����������ᣬʹ֮ת��Ϊ����������ˮ�У�������������������ζ���ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ���.
��5��������������ʱ�Ƚ�ʢ�л������Թܳ�����ñ���̼������Һ�кͻӷ����������ᣬʹ֮ת��Ϊ����������ˮ�У��ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����÷ֲ��ȡ�ϲ���������������Է���õ����������ķ����Ƿ�Һ��������Ҫ�IJ��������Ƿ�Һ©�����ձ���
��6��������Ӧ�ı���Ϊ�����ǻ��������⣬�������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ���÷�ӦΪ���淴Ӧ�����Է�Ӧ�Ļ�ѧ����ʽ��CH3COOH + CH3CH218OH CH3CO18OCH2CH3 +
H2O��
CH3CO18OCH2CH3 +
H2O��
(7)���������ǿ��̼��ģ����������̼�����Ʒ�Ӧ�Ļ�ѧ����ʽ��CH3COOH + NaHCO3 ��CH3COONa + CO2 �� + H2O��
���㣺���������������Ʊ�
�����������ǻ���������Ŀ��飬������۽̲Ļ���֪ʶ�������ǿ��ּ������ѧ���淶�Ͻ���ʵ����������Ͷ��ֲ��������������ڼ���ѧ����ѧϰ��Ȥ����ǿѧ����ѧϰ�����ġ����ʱ��ע��������Ӧ��ԭ���ͱ���̼������Һ�����ã���Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
����22�֣�
��4�֣�����ʵ������У���������
A��ʵ��������ϩʱ���ھƾ���Ũ����Ļ��Һ�У����뼸Ƭ���Ƭ�����Ȼ���ʹҺ���¶�Ѹ������170��
B����֤������ˮ�����ʱ���������������������Һ��ϣ��������Һ�����ã���Һ��ֲ�μ���������Һ
C����ͭ˿�������״���ھƾ����ϼ��ȱ�ں�����������ˮ�Ҵ��У�����Ҵ�����Ϊ��ȩ��ʵ��
D�������еμ�����ϡ��ˮ���������������鱽��
E����ҵ�ƾ���ȡ��ˮ�ƾ�ʱ���ȼ���ʯ��Ȼ������������뽫�¶ȼƵ�ˮ������뷴ӦҺ�У��ⶨ��ӦҺ�¶�
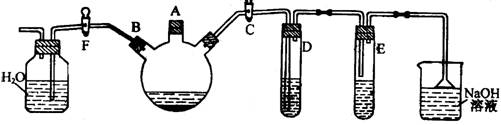
��10�֣�ʵ��������ͼ��ʾװ���Ʊ��屽������֤�÷�Ӧ��ȡ����Ӧ��
(1) �ر�F��������C��������װ��������������ƿ����A�ڼ��������壬�ټ���������м����סA�ڣ�������ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ�� ��
(2) D�Թ���װ���� ���������� ��
(3) E�Թ���װ���� ��E�Թ��ڳ��ֵ�����Ϊ ��
(4) ��������ƿ�еķ�Ӧ��������ʱ(��ʱ�������Լ���)����F�������ر�C���������Կ����������� ��
(5) ��һ���õ����屽��Ҫ�����²������ƣ�
a���� bˮϴ�� c�ø������� d 10%NaOH��Һϴ�ӣ� eˮϴ
��ȷ�IJ���˳����
��8�֣�������ͼ��ʾװ�ý���ʵ�飬��A��μ���B�У�
��1�� ��BΪNa2CO3��ĩ��CΪC6H5ONa��Һ��ʵ���й۲쵽С�Թ�����Һ�ɳ������ǣ����Թ�C�л�ѧ��Ӧ�����ӷ���ʽ��________________ ��Ȼ�����ձ��м����ˮ���ɹ۲쵽�Թ�C�е����� ��
��2�� ��B����ʯ�ң��۲쵽C��Һ�����γɳ�����Ȼ������ܽ�.��������ȫ�ܽ⣬ǡ�ñ����ʱ���ر�E.Ȼ����С�Թ��м���������ȩ��Һ�������ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������A�� �������ƣ���C�� ���ѧʽ��������ȩ�Ļ�Ϻ���Һ�з�Ӧ�Ļ�ѧ����ʽ�� _____________������D�ڴ�ʵ���е������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(14��)
ʵ��������ͼ��ʾװ���Ʊ��屽������֤�÷�Ӧ��ȡ����Ӧ�� www..com
(1) �ر�F��������C��������װ��������������ƿ����A�ڼ��������壬�ټ���������м����סA�ڣ�������ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ�� ��
(2) D�Թ���װ���� ���������� ��
(3) E�Թ���װ���� ��E�Թ��ڳ��ֵ�����Ϊ ��
(4) ��������ƿ�еķ�Ӧ��������ʱ(��ʱ�������Լ���)����F�������ر�C���������Կ����������� ��
(5) ��һ���õ����屽��Ҫ�����²������ƣ�a���� bˮϴ�� c�ø������� d 10%NaOH��Һϴ�ӣ� eˮϴ����ȷ�IJ���˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������ѧ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
����22�֣�
��4�֣�����ʵ������У��������� 
| A��ʵ��������ϩʱ���ھƾ���Ũ����Ļ��Һ�У����뼸Ƭ���Ƭ�����Ȼ���ʹҺ���¶�Ѹ������170�� |
| B����֤������ˮ�����ʱ���������������������Һ��ϣ��������Һ�����ã���Һ��ֲ�μ���������Һ |
| C����ͭ˿�������״���ھƾ����ϼ��ȱ�ں�����������ˮ�Ҵ��У�����Ҵ�����Ϊ��ȩ��ʵ�� |
| D�������еμ�����ϡ��ˮ���������������鱽�� |

 ϴ
ϴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
����22�֣�
��4�֣�����ʵ������У���������
A��ʵ��������ϩʱ���ھƾ���Ũ����Ļ��Һ�У����뼸Ƭ���Ƭ�����Ȼ���ʹҺ���¶�Ѹ������170��
B����֤������ˮ�����ʱ���������������������Һ��ϣ��������Һ�����ã���Һ��ֲ�μ���������Һ
C����ͭ˿�������״���ھƾ����ϼ��ȱ�ں�����������ˮ�Ҵ��У�����Ҵ�����Ϊ��ȩ��ʵ��
D�������еμ�����ϡ��ˮ���������������鱽��
E����ҵ�ƾ���ȡ��ˮ�ƾ�ʱ���ȼ���ʯ��Ȼ������������뽫�¶ȼƵ�ˮ������뷴ӦҺ�У��ⶨ��ӦҺ�¶�
��10�֣�ʵ��������ͼ��ʾװ���Ʊ��屽������֤�÷�Ӧ��ȡ����Ӧ��

(1) �ر�F��������C��������װ��������������ƿ����A�ڼ��������壬�ټ���������м����סA�ڣ�������ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ�� ��
(2) D�Թ���װ���� ���������� ��
(3) E�Թ���װ���� ��E�Թ��ڳ��ֵ�����Ϊ ��
(4) ��������ƿ�еķ�Ӧ��������ʱ(��ʱ�������Լ���)����F�������ر�C���������Կ����������� ��
(5) ��һ���õ����屽��Ҫ�����²������ƣ�
a���� bˮϴ�� c�ø������� d 10%NaOH��Һϴ�ӣ� eˮϴ
��ȷ�IJ���˳����
��8�֣�������ͼ��ʾװ�ý���ʵ�飬��A��μ���B�У�

��1�� ��BΪNa2CO3��ĩ��CΪC6H5ONa��Һ��ʵ���й۲쵽С�Թ�����Һ�ɳ������ǣ����Թ�C�л�ѧ��Ӧ�����ӷ���ʽ��________________ ��Ȼ�����ձ��м����ˮ���ɹ۲쵽�Թ�C�е����� ��
��2�� ��B����ʯ�ң��۲쵽C��Һ�����γɳ�����Ȼ������ܽ�.��������ȫ�ܽ⣬ǡ�ñ����ʱ���ر�E.Ȼ����С�Թ��м���������ȩ��Һ�������ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������A�� �������ƣ���C�� ���ѧʽ��������ȩ�Ļ�Ϻ���Һ�з�Ӧ�Ļ�ѧ����ʽ�� _____________������D�ڴ�ʵ���е������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ӱ�ʡ�߶���ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ�ʵ����
(14��)
ʵ��������ͼ��ʾװ���Ʊ��屽������֤�÷�Ӧ��ȡ����Ӧ�� www..com
(1) �ر�F��������C��������װ��������������ƿ����A�ڼ��������壬�ټ���������м����סA�ڣ�������ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ�� ��
(2) D�Թ���װ���� ���������� ��
(3) E�Թ���װ���� ��E�Թ��ڳ��ֵ�����Ϊ ��
(4) ��������ƿ�еķ�Ӧ��������ʱ(��ʱ�������Լ���)����F�������ر�C���������Կ����������� ��
(5) ��һ���õ����屽��Ҫ�����²������ƣ�a���� bˮϴ�� c�ø������� d 10%NaOH��Һϴ�ӣ� eˮϴ����ȷ�IJ���˳���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com