
 ��ͼ����װ���У�Һ�������Ϊ200mL����ʼ����ǰ�������Һ��Ũ�Ⱦ�Ϊ0.5mol/L������һ��ʱ������0.02mol����ͨ������������Һ����ı仯������������ȷ���ǣ�������
��ͼ����װ���У�Һ�������Ϊ200mL����ʼ����ǰ�������Һ��Ũ�Ⱦ�Ϊ0.5mol/L������һ��ʱ������0.02mol����ͨ������������Һ����ı仯������������ȷ���ǣ�������| A�� | ����������� ��=�� | |
| B�� | ���������������ӣ���������������С | |
| C�� | ��Һ��H+Ũ�ȱ仯�������ڼ�С | |
| D�� | �缫��Ӧʽ����������4OH--4e-�T2H2O+O2�������и�����2H++2e-�TH2�� |
���� ���ݢ��ǵ��أ��缫��ӦΪ��������4OH-��O2��+2H2O+4e-��������Cu2++2e-��Cu������ԭ��أ�������Ӧ��2H++2e-��H2��������Ӧ��Zn+2e-��Zn2+��Һ�������Ϊ200mL��Ũ�Ⱦ�Ϊ0.5mol/L����������ͭ����������ʵ�����Ϊ0.1mol�����ݵ����غ���������صļ��㣮
��� �⣺A�����ǵ��أ��缫��ӦΪ��������4OH-��O2��+2H2O+4e-��������Cu2++2e-��Cu������ԭ��أ�������Ӧ��2H++2e-��H2��������Ӧ��Zn+2e-��Zn2+��Һ�������Ϊ200mL��Ũ�Ⱦ�Ϊ0.5mol/L����������ͭ����������ʵ�����Ϊ0.1mol������0.02mol�ĵ���ͨ��ʱ���ٲ�����������������Ϊ0.005mol������0.01mol�����ų������Ԣ٣��ڣ���A����
B������������Ӧ��Cu2++2e-��Cu�������������ӣ�����������Ӧ��2H++2e-��H2�������������䣬��B����
C����Һ��pH�仯���������������������Լ��Լ���H+Ũ�����������������ӣ��������Լ���H+Ũ�ȼ�С����C��ȷ��
D���缫��Ӧʽ������������4OH--4e-=2H2O+O2�����и�����Zn-2e-��Zn2+����D����
��ѡC��
���� ���⿼��ѧ��ԭ��غ͵��صĹ���ԭ���Ծ��õ缫��Ӧ����д֪ʶ�����Ը�����ѧ֪ʶ���лش��ѶȲ���


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1 | B�� | 2 | C�� | 3 | D�� | 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ���ᴿ������ | ���� | �����Լ� | ���ӷ��� |
| A | CO2 ��g�� | SO2��g�� | ����Na2CO3��Һ��ŨH2SO4 | ϴ�� |
| B | NH4Cl��aq�� | Fe3+��aq�� | NaOH��Һ | ���� |
| C | NaCl��s�� | KNO3��s�� | AgNO3��Һ | ���� |
| D | Cu��s�� | Ag��s�� | CuSO4��Һ | ��ⷨ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
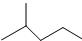 ��ʾ�ķ���ʽC6H14��������2-�����飬�˴Ź�����������5��壮
��ʾ�ķ���ʽC6H14��������2-�����飬�˴Ź�����������5��壮 �к��еĹ����ŵ�����Ϊ�ǻ���������
�к��еĹ����ŵ�����Ϊ�ǻ����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڲ�ͬ�¶��£�����ͬŨ����ͬ�����Na2S2O3��H2SO4��Ӧ��̽���¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죺3S2O32-+2SO42+10H+�T6SO2��+2S��+5H2O | |
| B�� | ���������£�KI��O2���������ӷ���ʽ��4I-+O2+4H+�T2I2+2H2O | |
| C�� | ��֪25�桢101kPaʱ��1molH2����������ȫ��Ӧ������̬HBr�ų�����Q kJ�����Ȼ�ѧ����ʽΪH2��g��+Br2��g���T2HBr��g����H=-2Q kJ•mol-1 | |
| D�� | ��ʾ�к��ȵ��Ȼ�ѧ����ʽ��2HCl��aq��+Ba��OH��2��aq���TBaCl2��aq��+2H2O��l����H=-114.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������CaCl2��Һ�������˰�ɫ����������Һ��һ�����д�����CO32- | |
| B�� | NaCl��Һ��Na2CO3��������ʱ������ɫ��ͬ | |
| C�� | ͨ�����ȵ�CuO����ʹ�����ɺڱ�������һ����H2 | |
| D�� | �����Ȼ�����Һ�а�ɫ�����������ټ����ᣬ��������ʧ��һ����SO42- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com