
���� �Ȼ�ѧ����ʽ2CH3OH��g��?CH3OCH3��g��+H2O��g����H=-24.5kJ•mol-1�ĺ�����2molCH3OH��ȫ�ֽ�ų���������24.5kJ������һ�������¼���2molCH3OH��һ��ʱ���������Ӧ��ƽ�⣬��ϵ�ų�����11kJ����ͬһ�����¼���0.2molCH3OCH3��0.2molH2O����ȫ�����CH3OH�����ʵ���Ϊ0.4mol���ں��º�ѹ�£�����2molCH3OH�����0.4molCH3OH���ڵ�Чƽ�⣬��ƽ��ʱCH3OH��ת������ͬ���ų����ȳ����ȹ�ϵ���ٸ�����������ȣ��淽�����ȣ��������ת�������淽��IJ���֮��Ϊ1���
��� �⣺�Ȼ�ѧ����ʽ2CH3OH��g��?CH3OCH3��g��+H2O��g����H=-24.5kJ•mol-1�ĺ�����2molCH3OH��ȫ�ֽ�ų���������24.5kJ������һ�������¼���2molCH3OH��һ��ʱ���������Ӧ��ƽ�⣬��ϵ�ų�����11kJ����ͬһ�����¼���0.2molCH3OCH3��0.2molH2O����ȫ�����CH3OH�����ʵ���Ϊ0.4mol��
�ں��º�ѹ�£�����2molCH3OH�����0.4molCH3OH���ڵ�Чƽ�⣬��ƽ��ʱCH3OH��ת������ͬ���ų����ȳ����ȹ�ϵ��$\frac{2mol}{0.4mol}$=$\frac{11kJ}{{Q}_{��}}$��Q��=2.2kJ��
����0.4molCH3OH��ͬһ������0.2molCH3OCH3��0.2molH2O���ڲ�ͬ�������ĵ�Чƽ�⣬0.4molCH3OH��ȫ�ֽ�ų�������Ϊ��$\frac{24.5kJ}{2mol}$��0.4mol=4.9kJ��
����CH3OH���ԣ���������ȣ��淽�����ȣ�������������ƽ����ת�������淽������ƽ�������֮��Ϊ1��
���淽������Ϊ4.9kJ-2.2kJ=2.7kJ��
�ʴ�Ϊ������2.7kJ��������
���� ���⿼�����Ȼ�ѧ����ʽ�ĺ��壬���淴Ӧ���ص㣬�ر������õ�Чƽ���������ǹؼ���ע�����⣬��Ŀ���Ѷȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ�Ӱ뾶��Z��R��W | |
| B�� | ��̬�⻯���ȶ��ԣ�HnW��HmR | |
| C�� | X2W6�����и�ԭ���������Ӿ�����8���ӽṹ | |
| D�� | Y��Z�γɵĻ�������ֻ�����Ӽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ϩ�Ľṹ��ʽ��CH2=CH2 | B�� | ����ķ���ʽ��CH3COOH | ||
| C�� | ����ı���ģ�ͣ�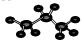 | D�� | ��������Ľṹʽ��CH3COOCH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ǡ����ۡ���֬����ˮ������Ϊ�ǵ���� | |
| B�� | ���������ã��������Ҵ����漰�������ܵ����� | |
| C�� | ������ˮ����NH4+��NH3�����û�ѧ��������绯ѧ���������� | |
| D�� | pH�ƿ���������к͵ζ��յ������������ˮ��̶ȵ��ж� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ֻ��ˮ���Լ���ױ������Ȼ�̼ | |
| B�� | CCl4����CH4�Ƶã�����ȡ��ˮ�еĵ� | |
| C�� | �Ҵ������в�����OH- | |
| D�� | 95%���Ҵ���Һ������ҽ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
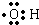 ����
����
| A�� | ֻ�Т� | B�� | ֻ�Тٺ͢� | C�� | ֻ�Тں͢� | D�� | �٢ڢۢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com