
a.ȡһ֧��ʽ�ζ��ܣ�b.������ˮϴ����c.��������NaOH��Һ��d.��¼Һ��̶ȵĶ�����e.����ʽ�ζ��ܾ�ȷ�ų�һ��������Һ��f.����δ������Һ��ϴ�Ľྻ��ƿ�У�g.������������ˮ��h.����2�η�̪��Һ��i.��ʼ�ζ����ߵα�ҡ����j.��ע�ӵζ�����Һ��仯��k.��С�ĵε���Һ����ɫ��ɷۺ�ɫʱ����ֹͣ�ζ���l.��¼Һ��̶ȵĶ�����m.���ݵζ��ܵ����ζ����ó�NaOH��Һ�����Ϊ22 mL��ָ������ʵ������еĴ���֮��(�ñ�ű�ʾ)_______________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
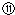 ��¼Һ��̶ȵĶ�����
��¼Һ��̶ȵĶ�����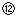 ���ݵζ��ܵ����ζ����ó�NaOH��Һ�����Ϊ22mL
���ݵζ��ܵ����ζ����ó�NaOH��Һ�����Ϊ22mL| ����NaOH��Һ�����V/mL | 0.00 | 18.00 | 19.80 | 19.98 | 20.00 | 20.02 | 20.20 | 22.00 | 40.00 |
| ʣ��������Һ�����V/mL | 20.00 | 2.00 | 0.20 | 0.02 | 0.00 | / | / | / | / |
| ����NaOH��Һ�����V/mL | / | / | / | / | / | 0.02 | 0.20 | 2.00 | 20.00 |
| pH | 1.00 | 2.28 | 3.30 | 7.00 | 9.70 | 11.70 | 12.50 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijѧ���к͵ζ�ʵ��Ĺ������£�(a)ȡһ֧��ʽ�ζ��ܣ�(b)������ˮϴ����(c)���������� NaOH��Һ��(d)��¼Һ��̶ȶ�����(e)����ʽ�ζ��ܾ�ȷ�ų�һ��������Һ��(f)����δ������Һ��ϴ�Ľྻ��ƿ�У�(g)������������ˮ��(h)�����̪��Һ2�Σ�(i)�ζ�ʱ���ߵα�ҡ����(j)��ע�ӵι���Һ��ı仯��(k)��С�ĵε���Һ����ɫ��ɷۺ�ɫʱ����ֹͣ�ζ���(l)��¼Һ��̶ȶ�����(m)���ݵζ��ܵ����ζ����ó�NaOH��Һ���Ϊ22mL��ָ������ʵ������еĴ���֮��(�ñ�ű�ʾ)___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�߶��ڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
ijѧ���к͵ζ�ʵ��Ĺ������£�
��a��ȡһ֧��ʽ�ζ��ܣ�
��b��������ˮϴ����
��c����������֪Ũ�ȵ�NaOH��Һ��
��d����¼Һ��̶ȶ�����
��e������ʽ�ζ��ܾ�ȷ�ų�һ�����������Һ��
��f������δ��������Һ��ϴ�Ľྻ��ƿ�У�
��g�������̪��Һ2�Σ�
��h���ζ�ʱ���ߵα�ҡ����
��i����ע�ӵζ�����Һ��ı仯��
��j����С�ĵε���Һ����ɫ��ɷۺ�ɫʱ����ֹͣ�ζ���
��k����¼Һ��̶ȶ�����
��l�����ݵζ��ܵ����ζ����ó�NaOH��Һ���Ϊ22mL��
����ʵ������еĴ���֮���У�
A����c����f����i����j�� B����c����f����i����j����l��
C����c����h����i����j�� D����c����i����j����l��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com