
 ����������������ϵ�д�
����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ?
? NH3?H2O
NH3?H2O NH4++OH-
NH4++OH- NH3?H2O
NH3?H2O NH4++OH-
NH4++OH-�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

����д���пհ�:
(1)д���������ʵĻ�ѧʽ:B____________________��C_______________��D_______________��K_______________��G_______________��J_______________��
(2)д�����з�Ӧ�����ӷ���ʽ:
��H+E(��Һ)��M_______________________________________________________��
��I����G_____________________________________________________________��
(3)��ͨ��״���£���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��д���������ʵĻ�ѧʽ��B____________��J____________��
��2��д�����з�Ӧ�����ӷ���ʽ��
��H+E����Һ����M_______________________________________________________��
��I����G���ּ���_______________________________________________________��
��3����ͨ��״���£���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ��ˮ�ж��и���ģ�⣨5�£����Ի�ѧ�Ծ����������� ���ͣ������
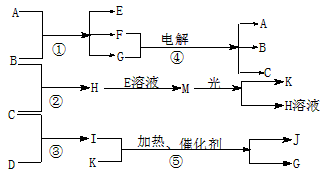
��1����ÿ��1�֣�����ÿ��2�֣���12�֣�����A�ǻ����B��C��D��K���ǵ��ʣ���Ӧ�ڡ��ݶ��dz����Ĺ�ҵ�����ķ�Ӧ�����й�����֮������Ӧת����ϵ����ͼ��ʾ��
����д���пհף�
(1)д���������ʵĻ�ѧʽ��B �� C �� D ��
K ��G ��J ��
(2)д�����з�Ӧ�����ӷ���ʽ��
��H+E(��Һ)��M ��
��I����G ��
(3)��ͨ��״���£���1 g ��������B������ȼ������H����ʱ�ų�92.3 k����������2 mol H������ȫ�ֽ�����C�����B������Ȼ�ѧ����ʽΪ
��
�鿴�𰸺ͽ���>>
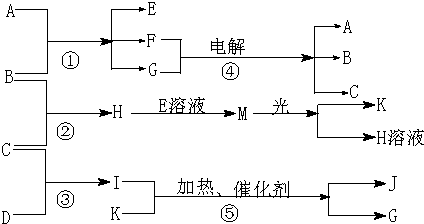
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ��ˮ�и���ģ�⣨5�£����Ի�ѧ�Ծ��������棩 ���ͣ��ƶ���
��1����ÿ��1�֣�����ÿ��2�֣���12�֣�����A�ǻ����B��C��D��K���ǵ��ʣ���Ӧ�ڡ��ݶ��dz����Ĺ�ҵ�����ķ�Ӧ�����й�����֮������Ӧת����ϵ����ͼ��ʾ��

����д���пհף�
(1)д���������ʵĻ�ѧʽ��B �� C �� D ��
K ��G ��J ��
(2)д�����з�Ӧ�����ӷ���ʽ��
��H+E(��Һ)��M ��
��I����G ��
(3)��ͨ��״���£���1 g ��������B������ȼ������H����ʱ�ų�92.3 k����������2 mol H������ȫ�ֽ�����C�����B������Ȼ�ѧ����ʽΪ
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com