
 ���ڹ�ҵ��չ��ȼ���豸�������࣬�豸��ģ����������Щ�����ŷŵ������ж����д�����SO2��������ͳ�ƣ��ҹ�1995�깤ҵSO2���ŷ���Ϊ1396��֣�2006�깤ҵSO2���ŷ����ﵽ��3800��֣�����SO2����Ⱦ���ҹ�ÿ����ʧ�ߴ�1100��Ԫ��
���ڹ�ҵ��չ��ȼ���豸�������࣬�豸��ģ����������Щ�����ŷŵ������ж����д�����SO2��������ͳ�ƣ��ҹ�1995�깤ҵSO2���ŷ���Ϊ1396��֣�2006�깤ҵSO2���ŷ����ﵽ��3800��֣�����SO2����Ⱦ���ҹ�ÿ����ʧ�ߴ�1100��Ԫ��| 2.72g |
| 136g/mol |
| 0.18g |
| 18g/mol |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
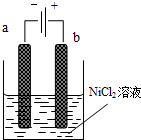 ������������Ni2O3��������������ܵ�أ�һ���Ʊ�Ni2O3�ķ����ǵ��NiCl2��Ni3+���ٽ�Ni3+��һϵ�з�Ӧ��ת��ΪNi2O3�������Ʊ��������£���NaOH��NiCl2��ҺpH��7.5���ӷ����������ƺ���е�⣮�������в�����Cl2������������������ClO-���Ѷ���������Ϊ����������ͼΪ���װ��ʾ��ͼ�����������������ӽ���Ĥ��������ش��������⣺
������������Ni2O3��������������ܵ�أ�һ���Ʊ�Ni2O3�ķ����ǵ��NiCl2��Ni3+���ٽ�Ni3+��һϵ�з�Ӧ��ת��ΪNi2O3�������Ʊ��������£���NaOH��NiCl2��ҺpH��7.5���ӷ����������ƺ���е�⣮�������в�����Cl2������������������ClO-���Ѷ���������Ϊ����������ͼΪ���װ��ʾ��ͼ�����������������ӽ���Ĥ��������ش��������⣺| �ŵ� |
| ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���ܱ���������A��B�������ʣ���һ���������·�Ӧ��2A������+B���̣�?2C��������H��0�ﵽƽ��ı�һ��������X������������Y��һ������ͼ�����ߵ��ǣ�������
���ܱ���������A��B�������ʣ���һ���������·�Ӧ��2A������+B���̣�?2C��������H��0�ﵽƽ��ı�һ��������X������������Y��һ������ͼ�����ߵ��ǣ�������| X | Y | |
| A | ��Сѹǿ | A��ת���� |
| B | �����¶� | �������ƽ�������� |
| C | �����¶� | A��Ũ�� |
| D | ����ѹǿ | B��ת���� |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| CO2 | H2��g�� | CO��g�� | H2O��g�� | |
| �� | a mol | a mol | 0 | 0 |
| �� | a mol | 2a mol | 0 | 0 |
| �� | a mol | a mol | 0 | a mol |
| A��n���ף���n���ң���n������ |
| B��n���ף���n��������n���ң� |
| C��n���ң���n��������n���ף� |
| D��n���ң���n���ף���n������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ���ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶�����
��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ���ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����pH=5��������Һϡ��500����Һ��c��SO42-����c��H+����1��10 |
| B�������£���pH=5�Ĵ�����Һϡ��100������ҺpH=7 |
| C�������£���pH=9������������Һϡ��100������ҺpH=7 |
| D�������£���0.01mol?L-1��������Һϡ��100������ҺpH=4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ |
| B���ϳ���ά |
| C�������մɺ�̼��ά�ĸ��ϲ��� |
| D��þ���Ͻ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������Ӧָ�������ʷ�������ˮ�� |
| B�����ά�ͺϳ���ά�������л��߷��Ӳ��� |
| C���������͡���ˮ�Ҵ����Ǵ����� |
| D��ú��������Һ��������ѧ�仯���̣��ɱ�Ϊ�����Դ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��һ������������1L������ͨ��46gNO2����NO2 �����ʵ���Ũ��һ��Ϊ1mol/L |
| B�������22.4L�ļ�������20NAԭ�� |
| C��1mol��������ˮת��1mol e- |
| D��1molNa2O2������ˮ��Ӧ��ת��1mol e-������0.5molO2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com