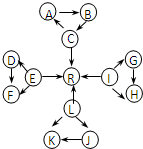
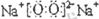ҪвЈәУЙKУлJҫщУЙ2ЦЦПаН¬өДФӘЛШЧйіЙЈ¬KОӘөӯ»ЖЙ«№ММеЈ¬ФтKОӘNa
2O
2Ј¬JОӘNa
2OЈ¬CЎўEЎўLЎўIОӘіЈјы№ММеөҘЦКЈ¬CЎўEЎўL¶јДЬУлЛ®ФЪТ»¶ЁМхјюПВ·ҙУҰЙъіЙЖшМеөҘЦКRЈ¬DЎўFЦРЛщә¬өД·ЗҪрКфФӘЛШЛщРОіЙөДөҘЦКіЈОВПВОӘ»ЖВМЙ«ЖшМеЈ¬ФтLОӘNaЈ¬RОӘH
2Ј¬EОӘFeЈ¬DЈЁFЈ©ОӘFeCl
2Ј¬FЈЁDЈ©ОӘFeCl
3Ј¬CОӘCЈ¬AОӘCOЈ¬BОӘCO
2Ј¬УЦCЎўLЎўIИэФӘЛШФӯЧУөДФӯЧУРтКэЦ®әНОӘ30Ј¬IөДФӯЧУРтКэОӘ30-11-6=13Ј¬ФтIОӘAlЈ¬GОӘAlЈЁOHЈ©
3Ј¬HОӘNaAlO
2Ј¬
ЈЁ1Ј©УЙЙПКц·ЦОцҝЙЦӘЈ¬RОӘЗвЖшЈ¬KОӘNa
2O
2Ј¬ЖдөзЧУКҪОӘ
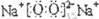
Ј¬№Кҙр°ёОӘЈәЗвЖшЈ»
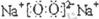
Ј»
ЈЁ2Ј©УЙBЙъіЙCөД»ҜС§·ҙУҰ·ҪіМКҪОӘ2Mg+CO
2
2MgO+CЈ¬№Кҙр°ёОӘЈә2Mg+CO
2
2MgO+CЈ»
ЈЁ3Ј©IәНЗвСх»ҜДЖИЬТә·ҙУҰөДАлЧУ·ҪіМКҪОӘ2Al+2OH
-+2H
2O=2AlO
2-+3H
2ЎьЈ¬HЛ®ИЬТәЛ®ҪвіКјоРФЈ¬ФтАлЧУ·ҙУҰОӘAlO
2-+2H
2O?AlЈЁOHЈ©
3+OH
-Ј¬
№Кҙр°ёОӘЈә2Al+2OH
-+2H
2O=2AlO
2-+3H
2ЎьЈ»AlO
2-+2H
2O?AlЈЁOHЈ©
3+OH
-Ј»
ЈЁ4Ј©¶иРФөзј«өзҪвNaClИЬТәЈ¬УЙ2NaClЎ«2NaOHЎ«2e
-Ј¬ФтНЁ№эөзЧУОӘ0.2molЈ¬nЈЁNaOHЈ©=0.2molЈ¬cЈЁNaOHЈ©=

=0.1mol/LЈ¬ЛщТФpH=13Ј¬№Кҙр°ёОӘЈә13Ј»
ЈЁ5Ј©ИфDИЬТәЦРә¬УРФУЦКFЈ¬іэФУ·Ҫ·ЁОӘИфDОӘFeCl
2Ј¬ФтНЁИлЧгБҝөДCl
2јҙҝЙЈ®ИфDОӘFeCl
3Ј¬ФтјУИлЧгБҝөДFeЈ¬ФЩ№эВЛЈ¬
№Кҙр°ёОӘЈәИфDОӘFeCl
2Ј¬ФтНЁИлЧгБҝөДCl
2јҙҝЙЈ®ИфDОӘFeCl
3Ј¬ФтјУИлЧгБҝөДFeЈ¬ФЩ№эВЛЈ®
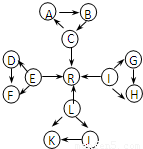
·ЦОцЈәУЙKУлJҫщУЙ2ЦЦПаН¬өДФӘЛШЧйіЙЈ¬KОӘөӯ»ЖЙ«№ММеЈ¬ФтKОӘNa
2O
2Ј¬JОӘNa
2OЈ¬CЎўEЎўLЎўIОӘіЈјы№ММеөҘЦКЈ¬CЎўEЎўL¶јДЬУлЛ®ФЪТ»¶ЁМхјюПВ·ҙУҰЙъіЙЖшМеөҘЦКRЈ¬DЎўFЦРЛщә¬өД·ЗҪрКфФӘЛШЛщРОіЙөДөҘЦКіЈОВПВОӘ»ЖВМЙ«ЖшМеЈ¬ФтLОӘNaЈ¬RОӘH
2Ј¬EОӘFeЈ¬DЈЁFЈ©ОӘFeCl
2Ј¬FЈЁDЈ©ОӘFeCl
3Ј¬CОӘCЈ¬AОӘCOЈ¬BОӘCO
2Ј¬УЦCЎўLЎўIИэФӘЛШФӯЧУөДФӯЧУРтКэЦ®әНОӘ30Ј¬IөДФӯЧУРтКэОӘ30-11-6=13Ј¬ФтIОӘAlЈ¬GОӘAlЈЁOHЈ©
3Ј¬HОӘNaAlO
2Ј¬И»әуҪбәПөҘЦКј°»ҜәПОпөДРФЦКАҙҪвҙрЈ®
өгЖАЈәұҫМвҝјІйОЮ»ъОпөДНЖ¶ПЈ¬Kј°CЎўEЎўL¶јДЬУлЛ®ФЪТ»¶ЁМхјюПВ·ҙУҰЙъіЙЖшМеөҘЦКRОӘҪвҙрұҫМвөДН»ЖЖҝЪЈ¬ЧўТвАыУГЧӘ»Ҝ№ШПөНЖ¶ПіцёчОпЦККЗҪвҙрөД№ШјьЈ¬КмПӨіЈјыөДөҘЦКј°»ҜәПОпөДРФЦКјҙҝЙҪвҙрЈ¬ЧўТв»щҙЎЦӘК¶өД»эАЫәН№йДЙЈ¬МвДҝДС¶ИЦРөИЈ®

 ФӘЛШј°Жд»ҜәПОпКЗЦРС§»ҜС§өДЦчёЙЦӘК¶Ј¬ОТГЗФЪХыАнФӘЛШ»ҜәПОпЦ®јдөДПа»ҘЧӘ»ҜКұЈ¬·ўПЦУРР©ОпЦКјдҙжФЪИзНјЛщКҫөДИэҪЗЧӘ»Ҝ№ШПөЈЁІҝ·Ц·ҙУҰОп»тЙъіЙОпТСВФИҘЈ©Јә
ФӘЛШј°Жд»ҜәПОпКЗЦРС§»ҜС§өДЦчёЙЦӘК¶Ј¬ОТГЗФЪХыАнФӘЛШ»ҜәПОпЦ®јдөДПа»ҘЧӘ»ҜКұЈ¬·ўПЦУРР©ОпЦКјдҙжФЪИзНјЛщКҫөДИэҪЗЧӘ»Ҝ№ШПөЈЁІҝ·Ц·ҙУҰОп»тЙъіЙОпТСВФИҘЈ©Јә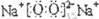 Ј¬№Кҙр°ёОӘЈәЗвЖшЈ»
Ј¬№Кҙр°ёОӘЈәЗвЖшЈ»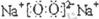 Ј»
Ј» 2MgO+CЈ¬№Кҙр°ёОӘЈә2Mg+CO2
2MgO+CЈ¬№Кҙр°ёОӘЈә2Mg+CO2 2MgO+CЈ»
2MgO+CЈ» =0.1mol/LЈ¬ЛщТФpH=13Ј¬№Кҙр°ёОӘЈә13Ј»
=0.1mol/LЈ¬ЛщТФpH=13Ј¬№Кҙр°ёОӘЈә13Ј»


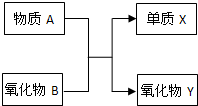 ФӘЛШј°Жд»ҜәПОпөДЦӘК¶КЗЎ°»ҜС§IЎұөДЦШөгДЪИЭЈ® AЎўBЎўXЎўYҫщОӘЦРС§ҪЧ¶ОөДіЈјыОпЦКЈ¬ЛьГЗЦ®јдөДЧӘ»Ҝ№ШПөИзНјЛщКҫЈә
ФӘЛШј°Жд»ҜәПОпөДЦӘК¶КЗЎ°»ҜС§IЎұөДЦШөгДЪИЭЈ® AЎўBЎўXЎўYҫщОӘЦРС§ҪЧ¶ОөДіЈјыОпЦКЈ¬ЛьГЗЦ®јдөДЧӘ»Ҝ№ШПөИзНјЛщКҫЈә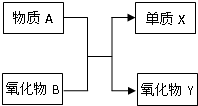 ФӘЛШј°Жд»ҜәПОпөДЦӘК¶КЗЎ°»ҜС§IЎұөДЦШөгДЪИЭЈ® AЎўBЎўXЎўYҫщОӘЦРС§ҪЧ¶ОөДіЈјыОпЦКЈ¬ЛьГЗЦ®јдөДЧӘ»Ҝ№ШПөИзНјЛщКҫЈә
ФӘЛШј°Жд»ҜәПОпөДЦӘК¶КЗЎ°»ҜС§IЎұөДЦШөгДЪИЭЈ® AЎўBЎўXЎўYҫщОӘЦРС§ҪЧ¶ОөДіЈјыОпЦКЈ¬ЛьГЗЦ®јдөДЧӘ»Ҝ№ШПөИзНјЛщКҫЈә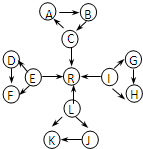 ФӘЛШј°Жд»ҜәПОпКЗЦРС§»ҜС§өДЦчёЙЦӘК¶Ј¬ОТГЗФЪХыАнФӘЛШ»ҜәПОпЦ®јдөДПа»ҘЧӘ»ҜКұЈ¬·ўПЦУРР©ОпЦКјдҙжФЪИзНјЛщКҫөДИэҪЗЧӘ»Ҝ№ШПөЈЁІҝ·Ц·ҙУҰОп»тЙъіЙОпТСВФИҘЈ©Јә
ФӘЛШј°Жд»ҜәПОпКЗЦРС§»ҜС§өДЦчёЙЦӘК¶Ј¬ОТГЗФЪХыАнФӘЛШ»ҜәПОпЦ®јдөДПа»ҘЧӘ»ҜКұЈ¬·ўПЦУРР©ОпЦКјдҙжФЪИзНјЛщКҫөДИэҪЗЧӘ»Ҝ№ШПөЈЁІҝ·Ц·ҙУҰОп»тЙъіЙОпТСВФИҘЈ©Јә