
Ϊ�ⶨ̼��ƴ��ȣ��躬����SiO2����ijѧ���о���ѧϰС����������¼���ʵ�鷽����
[������]��1����ȡ̼�����Ʒm g��2��������������3���ռ����ⶨ���ɵ��������V mL��[������]��1����ȡ̼�����Ʒm g����2����c mol/L����VmL���������ܽ���Ʒ����3��ȡ�ܽ�����Һ![]() mL����c��mol/L NaOH��Һ�ζ���ǡ����ȥV��Ml
mL����c��mol/L NaOH��Һ�ζ���ǡ����ȥV��Ml
[������]��1����ȡ̼�����Ʒm g����2�����£�1000�棩����ֱ���������ٷ����仯����ȴ�����������Ϊm��g��
[������] ��1����ȡ̼�����Ʒm g��
��2����������c mol / L����V mLʹ֮��ȫ�ܽ⣻
��3�����˲�ȡ��Һ��
��4������Һ�м������c��mol/L Na2CO3��ҺV��mL��
��5�������裨4���еij����˳���ϴ�ӡ��������Ϊm��g��
�ش��������⣺
��1������Ϊ����4�������У���õķ����� ������������ȱ��ֱ��ǣ�
���� ��
���� ��
���� ��
��2��Ϊ���ٷ�����ʵ������Ҫ�IJ����� ����ѡ���ţ���
A����ȷ�ⶨNa2CO3��Һ���V��mL
B����ȷ����Ũ��Ϊc��mol/L Na2CO3��Һ
C�������裨3�����ó���ϴ�ӣ�ϴ��ҺҲӦ���루4����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| V | 10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| V | 10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 Ϊ�ⶨ̼��ƴ��ȣ�����������ΪSiO2����ͬѧ���������������ʵ�鷽����
Ϊ�ⶨ̼��ƴ��ȣ�����������ΪSiO2����ͬѧ���������������ʵ�鷽�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꺣��ʡ�����и�����ѧ�ڸ߿����У�һ����ѧ���� ���ͣ�ʵ����
��9�֣�Ϊ�ⶨ̼��ƴ��ȣ�����������ΪSiO2����ͬѧ���������������ʵ�鷽����
������I��
��1����װ�������ⶨ�������������ʵ��װ�ã������������ԣ�
��2����ȡ̼�����ƷW g��
��3������������
��4���ռ����ⶨ���ɵ��������V mL��
����I��1�����������Ʒʱ������ײ���һ��δ��ʵ���߷��ֵIJ�ȱ����ô�ⶨ��̼��
�ƵĴ��Ȼ� ���ƫ�ߡ�ƫ�͡���Ӱ�족����
����I��2���ڷ����л����ⶨ���ɵ����������װ�ü�ͼ��

������II��
��1����ȡ̼�����ƷWg��
��2������ƿ����C mol/L����V mL���������ܽ���Ʒ��
��3�����������ָʾ��������Ũ��ΪC1 mol/L�ı�����������Һ�ζ����������ᣬ��ȥ����������ҺV1����������֪���ȵı�ɫ��Χ��pH=3.1~4.4��
����II��1��δ��ȥ������SiO2���Բⶨ����Ƿ���Ӱ�죿�����л��ޣ� ��
������ ��
����II��2��̼��ƴ��ȱ���ʽ ��
���������ۡ�
����Ϊ�������������У���õķ����� ��
��һ�����������õ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��㶫ʡ����10���¿���ѧ���� ���ͣ�ʵ����
��16�֣�Ϊ�ⶨ̼��ƴ��ȣ��躬����SiO2����ѧ����������¼���ʵ�鷽������ش�ÿ����������������⡣
������I��
��1����ȡ̼�����Ʒ M g��
��2������������
��3���ռ����ⶨ���ɵ�������� V mL��
����1����Ӧ�������������е���ʾ����ͼ��ʾ���������IJ����ǣ� ����������Һ��Ķ�����
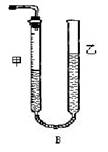
������II��
��1����ȡ̼�����Ʒ M g��
��2����c mol/L ���� V mL���������ܽ���Ʒ��
��3��ȡ�ܽ�����Һ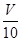 mL���Է�̪��ָʾ������c�� mol/L NaOH��Һ�ζ���ǡ����ȥV��mL��
mL���Է�̪��ָʾ������c�� mol/L NaOH��Һ�ζ���ǡ����ȥV��mL��
����2���г���ʵ�������õ�����Ҫ��������(������̨���������ձ�֮��) ��
����3��̼��ƴ��ȼ��㹫ʽ ��
����4����̼����ܽ���ȫ������δ�ܵ�SiO2û����ȥ������õ�̼��ƴ���________ (ƫ��ƫС����Ӱ�죩
��������
��1����ȡ̼�����Ʒ M g��
��2����������c mol/L����V mLʹ֮��ȫ�ܽ⣻
��3�����˲�ȡ��Һ��
��4������Һ�м������c�� mol/L Na2CO3��ҺV��mL��
��5�������裨4���еij����˳���ϴ�ӡ��������ΪM��g��
����5���˷����в���Ҫ�������� ����ѡ���ţ���
A��c��V B��c�䡢 V�� C�� M�� D�� M
����6��Ϊ����ʵ�������裨3������5�����˺�Ҫ�Գ�������ϴ�ӣ�������裨3��δ��ϴ�ӣ���ⶨ��̼��ƵĴ��Ƚ� ��ƫ��ƫС����Ӱ�죬��ͬ����������裨5��δ��ϴ�ӣ���ⶨ��̼��ƴ��Ƚ� ��
����7���жϲ��裨4����Na2CO3��Һ�Ƿ�����ķ����� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com