
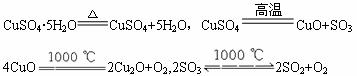
��ȡ25.0 g CuSO4��5H2O������ȣ�ʹ֮���ȡ�����������1000 �棬������1 h��������������⣺
(1)�������ù������ɫΪ____________������Ϊ____________ g��
(2)������ʵ����������ڷ�Ӧ�����£���Ӧ��ij�ȥˮ�����̬��������ʵ�������Ϊ____________(��д����ѡ��)��
A.0 B.0.1 mol C.����0.1 mol
(3)(2)���г���������ѡ���⣬����Ϊ��ȷ�ķ�ΧӦ����ʲô��������˵����
(4)���ijͬѧ����ʵ��ʱ������������Ϊ7.6 g����ͨ�������жϸù������ʵ���ɣ������ʵ������Ƕ��٣�
(1)��(��ש��)ɫ 7.2 (2)C
(3)CuO�ֽ�����0.025 mol O2��SO3�ֽ��ǿ��淴Ӧ����SO3�ֽⷴӦ��������0.1 mol��С��0.15 mol����������������ʵ�������0.125 mol��С��0.175 mol
(4)ʣ�����7.6 g������7.2��8.0 g֮�䣬��ΪCuO��Cu2O����
n(Cu)=n(CuSO4��5H2O)=0.1 mol
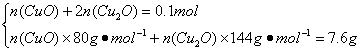
n(CuO)=0.05 mol
n(Cu2O)=0.025 mol
�������������Ϣ֪����Ӧ���չ�������Ǻ�ɫ��Cu2O������CuԪ���غ��m(Cu2O)= ![]() n(CuSO4��5H2O)��M(Cu2O)=
n(CuSO4��5H2O)��M(Cu2O)=![]() ��0.1 mol��144 g��mol-1=7.2 g������������0.1 mol CuSO4�ֽ����SO3��CuO��0.1 mol��CuO�ַֽ�����0.025 mol O2������SO3�ֽ��ǿ��淴Ӧ����Ӧ��������0.1 mol��С��0.15 mol����������������ʵ�������0.125 mol��С��0.175 mol������0.1 mol CuSO4��5H2O���·ֽ�ɵ�0.1 mol CuO��������Ϊ8.0 g��CuO�����ֽ����տɵ�7.2 g Cu2O���������������Ϊ7.6 g������7.2 g��8.0 g֮�䣬˵����CuO��Cu2O�Ļ���������CuԪ���غ��з�������������ʵ�����
��0.1 mol��144 g��mol-1=7.2 g������������0.1 mol CuSO4�ֽ����SO3��CuO��0.1 mol��CuO�ַֽ�����0.025 mol O2������SO3�ֽ��ǿ��淴Ӧ����Ӧ��������0.1 mol��С��0.15 mol����������������ʵ�������0.125 mol��С��0.175 mol������0.1 mol CuSO4��5H2O���·ֽ�ɵ�0.1 mol CuO��������Ϊ8.0 g��CuO�����ֽ����տɵ�7.2 g Cu2O���������������Ϊ7.6 g������7.2 g��8.0 g֮�䣬˵����CuO��Cu2O�Ļ���������CuԪ���غ��з�������������ʵ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��ϸ�Ķ���CuSO4��5H2O������ȹ��������η��������·�Ӧ��
CuSO4��5H2O![]() CuSO4+5H2O��CuSO4
CuSO4+5H2O��CuSO4![]() CuO+SO3
CuO+SO3
4CuO![]() 2Cu2O+O2,2SO3
2Cu2O+O2,2SO3![]() 2SO2+O2
2SO2+O2
��ȡ25.0 g CuSO4��5H2O������ȣ�ʹ֮���ȡ�����������1000 �棬������1 h��������������⣺
(1)�������ù������ɫΪ____________������Ϊ____________ g��
(2)������ʵ����������ڷ�Ӧ�����£���Ӧ��ij�ȥˮ�����̬��������ʵ�������Ϊ____________(��д����ѡ��)��
A.0 B.0.1 mol C.����0.1 mol
(3)(2)���г���������ѡ���⣬����Ϊ��ȷ�ķ�ΧӦ����ʲô��������˵����
(4)���ijͬѧ����ʵ��ʱ������������Ϊ7.6 g����ͨ�������жϸù������ʵ���ɣ������ʵ������Ƕ��٣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com