
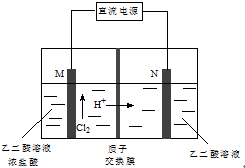
 ŅŅČ©Ėį£ØHOOC-CHO£©ŹĒÓŠ»śŗĻ³ÉµÄÖŲŅŖÖŠ¼äĢ壮¹¤ŅµÉĻÓĆ”°Ė«¼«ŹŅ³É¶Ōµē½ā·Ø”±Éś²śŅŅČ©Ėį£¬ŌĄķČēĶ¼ĖłŹ¾£®øĆ×°ÖĆÖŠŅõ”¢ŃōĮ½¼«ĪŖ¶čŠŌµē¼«£¬Į½¼«ŹŅ¾łæɲśÉśŅŅČ©Ėį£¬ĘäÖŠŅŅ¶žČ©ÓėMµē¼«µÄ²śĪļ·“Ӧɜ³ÉŅŅČ©Ėį£®ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
ŅŅČ©Ėį£ØHOOC-CHO£©ŹĒÓŠ»śŗĻ³ÉµÄÖŲŅŖÖŠ¼äĢ壮¹¤ŅµÉĻÓĆ”°Ė«¼«ŹŅ³É¶Ōµē½ā·Ø”±Éś²śŅŅČ©Ėį£¬ŌĄķČēĶ¼ĖłŹ¾£®øĆ×°ÖĆÖŠŅõ”¢ŃōĮ½¼«ĪŖ¶čŠŌµē¼«£¬Į½¼«ŹŅ¾łæɲśÉśŅŅČ©Ėį£¬ĘäÖŠŅŅ¶žČ©ÓėMµē¼«µÄ²śĪļ·“Ӧɜ³ÉŅŅČ©Ėį£®ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©| A£® | M¼«ÓėÖ±Į÷µēŌ“µÄøŗ¼«ĻąĮ¬ | |
| B£® | ČōÓŠ2 molH+ĶعżÖŹ×Ó½»»»Ä¤²¢ĶźČ«²ĪÓė·“Ó¦£¬ŌņøĆ×°ÖĆÖŠÉś³ÉµÄŅŅČ©ĖįĪŖ1 mol | |
| C£® | Nµē¼«ÉĻµÄµē¼«·“Ó¦Ź½£ŗHOOC-COOH-2e-+2H+=HOOC-CHO+H2O | |
| D£® | ŅŅ¶žČ©ÓėMµē¼«µÄ²śĪļ·“Ӧɜ³ÉŅŅČ©ĖįµÄ»Æѧ·½³ĢŹ½£ŗCl2+OHC-CHO+H2O=HOOC-CHO+2HCl |
·ÖĪö A”¢øł¾ŻÖŹ×ÓµÄŅĘ¶Æ·½Ļņ£¬Č·¶ØMµē¼«ŹĒŃō¼«£»
B”¢2mol H+ĶعżÖŹ×Ó½»»»Ä¤£¬Ōņµē³ŲÖŠ×ŖŅĘ2molµē×Ó£¬øł¾Żµē¼«·½³ĢŹ½¼ĘĖć£»
C”¢Nµē¼«ÉĻHOOC-COOHµĆµē×ÓÉś³ÉHOOC-CHO£»
D”¢ĀČĘų¾ßÓŠŃõ»ÆŠŌ£¬Äܽ«Č©»łŃõ»ÆĪŖōČ»ł£¬¾Ż“ĖŹéŠ“·½³ĢŹ½¼“æÉ£®
½ā“š ½ā£ŗA”¢øł¾ŻÖŹ×ÓµÄŅĘ¶Æ·½Ļņ£¬Č·¶ØMµē¼«ŹĒŃō¼«£¬M¼«ÓėÖ±Į÷µēŌ“µÄøŗ¼«ĻąĮ¬£¬¹ŹA“ķĪó£»
B”¢2mol H+ĶعżÖŹ×Ó½»»»Ä¤£¬Ōņµē³ŲÖŠ×ŖŅĘ2molµē×Ó£¬øł¾Żµē¼«·½³ĢŹ½HOOC-COOH+2e-+2H+ØTHOOC-CHO+H2O£¬æÉÖŖÉś³É1molŅŅČ©Ėį£¬ÓÉÓŚĮ½¼«¾łÓŠŅŅČ©ĖįÉś³ÉĖłŅŌÉś³ÉµÄŅŅČ©ĖįĪŖ2mol£¬¹ŹB“ķĪó£»
C”¢Nµē¼«ÉĻHOOC-COOHµĆµē×ÓÉś³ÉHOOC-CHO£¬Ōņµē¼«·“Ó¦Ź½ĪŖHOOC-COOH+2e-+2H+ØTHOOC-CHO+H2O£¬¹ŹC“ķĪó£»
D”¢ĀČĘų¾ßÓŠŃõ»ÆŠŌ£¬Äܽ«Č©»łŃõ»ÆĪŖōČ»ł£¬ŅŅ¶žČ©ÓėMµē¼«µÄ²śĪļ·“Ӧɜ³ÉŅŅČ©ĖįµÄ»Æѧ·½³ĢŹ½ĪŖ£ŗCl2+OHC-CHO+H2OØTHOOC-CHO+2HCl£¬¹ŹDÕżČ·£®
¹ŹŃ”D£®
µćĘĄ ±¾Ģāæ¼²éĮĖµē½ā³ŲŌĄķµÄ·ÖĪöÓ¦ÓĆ£¬°ŃĪÕµē½ā³ŲŌĄķŅŌ¼°µē½ā¹ż³ĢÖŠµē×ÓŹŲŗćµÄ¼ĘĖćÓ¦ÓĆ£¬ÕĘĪÕ»ł“”ŹĒ¹Ų¼ü£¬ĢāÄæÄѶČÖŠµČ£®


 ŗ®¼Ł““ŠĀŠĶ×ŌÖ÷ѧĻ°µŚČżŃ§ĘŚŗ®¼ŁĻĪ½ÓĻµĮŠ“š°ø
ŗ®¼Ł““ŠĀŠĶ×ŌÖ÷ѧĻ°µŚČżŃ§ĘŚŗ®¼ŁĻĪ½ÓĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | C1£¾C2 | |
| B£® | Į½ĘæČÜŅŗµÄµ¼µēÄÜĮ¦ĻąĶ¬ | |
| C£® | µŚŅ»ĘæČÜŅŗµÄpH“óÓŚµŚ¶žĘæČÜŅŗµÄpH | |
| D£® | µŚŅ»ĘæČÜŅŗÖŠ“×ĖįµÄµēĄė³Ģ¶ČŠ”ÓŚµŚ¶žĘæČÜŅŗÖŠ“×ĖįµÄµēĄė³Ģ¶Č |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŗģÉ«”¢ŗŚÉ«”¢°×É« | B£® | ŗģÉ«”¢°×É«”¢°×É« | C£® | ŗģÉ«”¢ŗģÉ«”¢ŗģÉ« | D£® | ŗŚÉ«”¢ŗģÉ«”¢°×É« |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | c£ØH+£©£¾c£ØF-£© | B£® | c£ØH+£©£¾c£ØHF£© | C£® | c£ØOH-£©£¼c£ØHF£© | D£® | c£ØHF£©£¾c£ØF-£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Na2O | B£® | NaCl | C£® | H2O | D£® | NaOH |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1.4g | B£® | 2.8g | C£® | 5.6g | D£® | 11.2g |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com