
���� ��1������ʵ������IJ��裨�������ȡ���ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��ǩ���Լ�ÿ��������Ҫ����ȷ����Ӧ�����������ò�����������
��2������c=$\frac{1000�Ѧ�}{M}$����ŨŨ��������ʵ���Ũ�ȣ�����ϡ�Ͷ��ɣ�CŨVŨ=CϡVϡ��������ҪŨ���������
��3������һ�����ʵ���Ũ����Һ��һ�㲽�裺�������ȡ���ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��ǩ���ݴ�����
����
��4�����Ǵ����������ʵ��ʧ���Ҳ��ܲ��ȵģ��������������ƣ�
��5����������ƿ���켰ʹ��ע��������
��6���������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1����Һ����һ�㲽���ǣ�������������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��ǩ��һ������Ͳ��ȡ�����ձ����ܽ⣬���ò��������裬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ת����ϣ�����������ˮϴ���ձ���������2��3�β���ϴ��Һȫ��ת�Ƶ�����ƿ�У��ټ���������ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ������ƿ�����������µߵ�ҡ�ȣ�
������Ҫ������Ϊ����Ͳ���ձ�����������500mL����ƿ����ͷ�ιܣ��ò�������������ƿ��������ƽ��ҩ�ף�
�ʴ�Ϊ���ڢߢࣻ
��2������c=$\frac{1000�Ѧ�}{M}$��֪Ũ�����Ũ��C=$\frac{1000��1.84��98%}{98}$=18.4mol/L��
����ҪŨ��������ΪVml������ϡ�Ͷ���CŨVŨ=CϡVϡ��֪�У�18.4mol/L��Vml��10-3=0.5mol/L��0.5L
��ã�V=13.6ml��
�ʴ�Ϊ��13.6ml��
��3������һ�����ʵ���Ũ����Һ��һ�㲽�裺�������ȡ���ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��ǩ��������ȷ��˳��Ϊ���ڢ٢ܢۢޢݣ�
��4�����ڲ������������Т�ʱ��ʹҺ���Գ���������ƿ�Ŀ̶ȣ����ߣ�����Һ���ƫ���������ȣ����Ա����������ƣ�
�ʴ�Ϊ���������ƣ�
��5��A������ƿ�ϱ����ݻ����¶ȡ����ߣ�����ѹǿ����A����
B������ƿΪ�������������ܳ�ʱ�������Լ�����B��ȷ��
C��Ϊʹʵ���ȷ������ƿϴ���������Ƶ���Һ��ϴ���������ʵ����ʵ���ƫ����ҺŨ��ƫ��C����
D��������ƿ������Һ����Ϊ����ʱ��Ȼ��Ҫ��������ˮ����������ƿʹ��ǰ��������D����
��ѡ��B��
��6��A��Ũ����ϡ�ͺ�δ�������¼����ж��ݣ���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ���Aѡ��
B������ƿδ���T����������Һ�������ʵ����ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣬��B��ѡ��
C��������ƿ��ת����Һʱ��������Һ�彦��������������ʧ�����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���C��ѡ��
D��ҡ�Ⱥ���Һ����ڿ̶��ߣ�û��ȡ�κδ�ʩ��������ȷ��������ҺŨ��ȷ����D��ѡ��
E������ͲȡŨ����ʱ���ӿ̶��ߣ�������ȡ��Ũ�������ƫС����������ʵ���ƫС����ҺŨ��ƫ�ͣ���E��ѡ��
F������ʱ�����ӿ̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���Fѡ��
��ѡ��AF��
���� ������Ҫ����������һ�����ʵ���Ũ����Һ�IJ��������������������Ϥ���ƹ��̺�ԭ���ǽ���ؼ���ע���������ķ���������ƿʹ�õ�ע�����


 һ����������ϵ�д�
һ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ˮ��Һ�У�H+��I-��NO3-��SiO32- | |
| B�� | ������ˮ�У�Cl-��NO3-��Na+��SO32- | |
| C�� | ������Һ�У�NO3-��I-��Na+��Fe2+ | |
| D�� | ������CO2ͨ��ʱ��H+��NH4+��Al3+��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������������ѧ�߶��ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ������
A����֪�����Ȼ�ѧ����ʽ��
��CH3COOH(l)��2O2(g)=2CO2(g)��2H2O(l) ��H1����870.3 kJ/mol
��C(s)��O2(g)= CO2(g) ��H2����393.5 kJ/mol
��H2(g)��1/2O2(g)=H2O(l) ��H3����285.8 kJ/mol
��Ӧ��2C(s)��2H2(g)��O2(g)=CH3COOH(l)���ʱ�Ϊ
b .��H2(g)��1/2O2(g)=H2O(l) ��H����285��8 kJ/mol
.��H2(g)��1/2O2(g)=H2O(l) ��H����285��8 kJ/mol
��H2(g)��1/2O2(g)=H2O(g) ��H����241��8 kJ/mol
��C(s)��1/2O2(g)=CO(g) ��H����110��5 kJ/mol
��C(s)��O2(g)=CO2(g) ��H����393��5 kJ/mol
�ش��������⣺
��1��������Ӧ�����ڷ��ȷ�Ӧ���� ��
��2��H2��ȼ����Ϊ ��C��ȼ����Ϊ ��
��3��CO��ȼ����Ϊ �����Ȼ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������и�һ��9�µ��л�ѧ�Ծ��������棩 ���ͣ�ʵ����
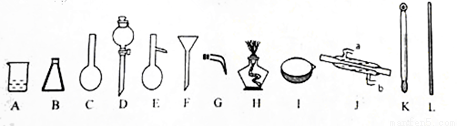
��1����д�������������ƣ�B D E
��2�������Ȼ�̼��ȡ����ˮ��Br2�ķ����ǣ� ����Br2�����Ȼ�̼��Һ��ˮ���뿪�IJ����ǣ�
��3����֪Br2�ķе���58.5�棬���Ȼ�̼�е���78�档��Br2�����Ȼ�̼��Һ�е�Br2��������IJ����� ���ò�����Ҫ�õ��IJ��������У�����ĸ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��Ϊ�ⶨ�����Ũ�Ƚ���ʵ�飮����д���пհף�
ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��Ϊ�ⶨ�����Ũ�Ƚ���ʵ�飮����д���пհף�ʵ���� | NaOH��Һ��Ũ��/��mol•L-1�� | �ζ����ʱ��NaOH��Һ��������/mL | ������������/mL |
| 1 | 0.10 | 22.62 | 20.00 |
| 2 | 0.10 | 22.72 | 20.00 |
| 3 | 0.10 | 22.80 | 20.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaOH | B�� | HCl | C�� | CaCO3�����壩 | D�� | H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ӦCO2��g��+C��s��=2CO��g���ڸ����¿��Է����У���÷�Ӧ�ġ�H��0 | |
| B�� | ��ҵ�ϵ�ⱥ��ʳ��ˮʱ����ʯī������������������ | |
| C�� | �����£�0.1mol•L-1 CH3COOH��ҺpH=1 | |
| D�� | �����£���AgCl����Һ�м�������NaCl������Һ��c��Ag+����С��Ksp��AgCl�� ��С����Һ��pH������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com