
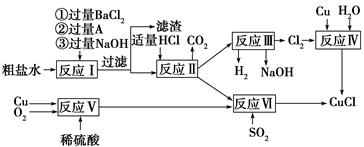



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®·½·Ø¢ŪŹĒ×ī¾¼ĆŗĶ»·±£µÄ |
| B£®·½·Ø¢ŁÖŠ£¬Čō“Ó¾¼ĆŗĶ»·±£µÄ½Ē¶Čæ¼ĀĒ£¬ÓĆĻ”ĻõĖį±ČÓĆÅØĻõĖįŗĆ |
| C£®·½·Ø¢ŚŠčŅŖĻūŗÄÄÜŌ“£¬¶Ō»·¾³²»²śÉśĪŪČ¾ |
| D£®·½·Ø¢ŪÖŠN2O4¼ČŹĒŃõ»Æ¼ĮÓÖŹĒ»¹Ō¼Į |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

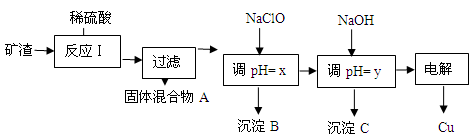
| ³ĮµķĪļ | Cu(OH)2 | Al(OH)3 | Fe(OH)3 | Fe(OH)2 |
| æŖŹ¼³ĮµķpH | 5.4 | 4.0 | 1.1 | 5.8 |
| ³ĮµķĶźČ«pH | 6.7 | 5.2 | 3.2 | 8.8 |
 HClO£¬ClO£ĻūŗÄH+£¬“Ó¶ų“ļµ½µ÷½ŚpHµÄÄæµÄ
HClO£¬ClO£ĻūŗÄH+£¬“Ó¶ų“ļµ½µ÷½ŚpHµÄÄæµÄ HClO+OH££¬OH£ĻūŗÄH+ £¬“Ó¶ų“ļµ½µ÷½ŚpHµÄÄæµÄ
HClO+OH££¬OH£ĻūŗÄH+ £¬“Ó¶ų“ļµ½µ÷½ŚpHµÄÄæµÄ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
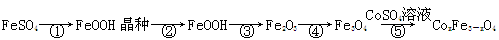
| ½šŹōĄė×Ó | æŖŹ¼³ĮµķµÄpH | ³ĮµķĶźČ«µÄpH |
| Fe3£« | 1.1 | 3.2 |
| Fe2£« | 5.8 | 8.8 |
| Co2£« | 6.9 | 9.4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
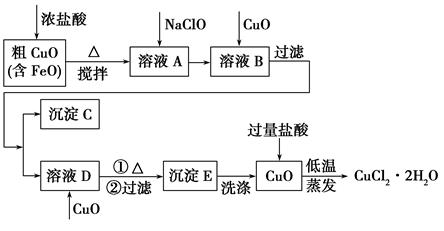
| ĪļÖŹ | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
| ĶźČ«³ĮµķŹ±µÄpH | ”Ż9.6 | ”Ż6.4 | 3”«4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
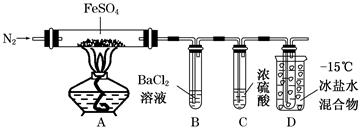
| ŹµŃé | ŹµŃé¹ż³Ģ | ŹµĻÖĻÖĻó |
| ¢Ł | ĶØČėŅ»¶ĪŹ±¼äN2£¬¼ÓČČ | AÖŠ¹ĢĢå±äĪŖŗģ×ŲÉ«£¬BÖŠÓŠ°×É«³Įµķ£¬DŹŌ¹ÜÖŠÓŠĪŽÉ«ŅŗĢå |
| ¢Ś | ÓĆ“ųÓŠ»šŠĒµÄľĢõææ½ü×°ÖĆDµÄµ¼¹ÜæŚ | ľĢõø“Č¼ |
| ¢Ū | ³ä·Ö·“Ó¦£¬Ķ£Ö¹¼ÓČČ£¬ĄäČ“ŗó£¬Č”AÖŠ¹ĢĢ壬¼ÓŃĪĖį | ¹ĢĢåČܽā£¬ČÜŅŗ³Ź»ĘÉ« |
| ¢Ü | ½«¢ŪĖłµĆČÜŅŗµĪČėDŹŌ¹ÜÖŠ | ČÜŅŗ±äĪŖĒ³ĀĢÉ« |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com