
��ȩ��ʳƷ��ҽҩ�������ȷ��涼��Ӧ�á����������dz����ڵ��ƾ��в�ݮ�����ѡ�ӣ�ҡ�����������ζ��ʳ���㾫��
��1�����ȩ��C��H��O����Ԫ����ɣ����������ȩ���ӵ���Է�������Ϊ132���������̼Ԫ�ص���������Ϊ81��8%�����ȩ�ķ���ʽ�� ��
���ȩ�DZ���һȡ����˴Ź���������ʾ�����������������ֲ�ͬ��ѧ��������ԭ�ӣ���ṹ��ʽ�� ����������˳���칹���ӳ�칹��
��2����֪��
I��ȩ��ȩ�ܷ�����Ӧ��ԭ�����£�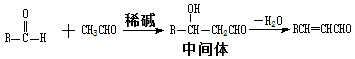
II���ϳ����ȩ�Ĺ�ҵ��������ͼ��ʾ�����м�Ϊij������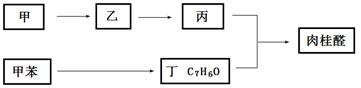
�ټĽṹ��ʽ�� ��
�ڱ��Ͷ��������Ļ�ѧ����ʽ�� ��
��3�����ȩ�ܱ�������Һ�������پ��ữ�õ�����ᣬд�����������Ľṹ��ʽ ��
��4�����÷�����AΪԭ�Ϻϳ���������H��·�����£����A�ĺ˴Ź���������ͼ��6���壬�����֮��Ϊ1�U2�U2�U2�U1�U2����
�ٻ�����F�еĹ������� �������ƣ���
��B��C�ķ�Ӧ������ ��F��G�ķ�Ӧ������ ��
����д��ѧ����ʽ
F��I
G��H
��G��ͬ���칹���У���������Ŀ������ֻ��һ��ȡ������ͬ���칹���� �֡�
��ṹ��ʽ�ֱ���
��1��C9H8O ��1�֣� 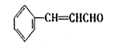 ��2�֣�
��2�֣�
��2����CH2=CH2��2�֣�
��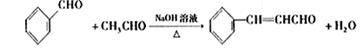 ��2�֣�
��2�֣�
��3��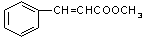 ��2�֣�
��2�֣�
��4�����ǻ����Ȼ� ��2�֣�
��ˮ�ⷴӦ����ȡ����Ӧ������ȥ��Ӧ ����1�֣�
��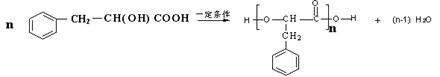 ��2�֣�
��2�֣� ��2�֣�
��2�֣�
�� 4 ��1�֣�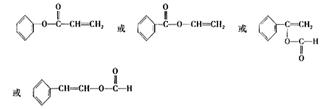 ��2�֣�
��2�֣�
���������������1�����ȩ����Է�������Ϊ132���������̼Ԫ�ص���������Ϊ81��8%�������ȩ�ķ����к�9��̼ԭ�ӣ�ȩ������Է�������Ϊ29����ʣ�µ�����ӦΪ103������������8��̼ԭ�ӣ�������������ԭ����7���������ȩ�ķ���ʽΪC9H8O�����ȩ�DZ���һ����҂�������3����ԭ�ӣ����к�ȩ�������Խṹ��ʽΪ
��2������ȩ��ȩ�ķ�Ӧԭ����֪�����ȩӦ�ɱ���ȩ����ȩ��Ӧ�õ������Ϊ��ȩ��������������������ȩ�����Լ�Ӧ����ϩ��ˮ�����Ҵ�������������ȩ����ϩ�Ľṹ��ʽΪCH2=CH2
����ȩ�뱽��ȩ��Ӧ�Ļ�ѧ����ʽ������Ŀ�е���֪���ɵó���Ϊ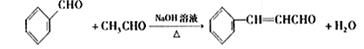
��3��������Һֻ����ȩ�����������������Ľṹ��ʽ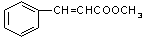
��4����A�ĺ˴Ź���������ͼ��6���壬�����֮��Ϊ1�U2�U2�U2�U1�U2���ж�A�е�̼̼˫���ڂ����Ķ�λ���ɴ��ж�A��B�����ӳɷ�Ӧ��B��C����ˮ�ⷴӦ��C��D����������Ӧ��D��E����������Ӧ��E��F�����ʻ����ⷴӦ������D�к��Ȼ����ǻ���
���ɷ�Ӧ�����ж�B��C�ķ�Ӧ������ˮ�ⷴӦ��F��G�ķ�Ӧ��������ȥ��Ӧ��
��F��I�������۷�Ӧ����ѧ����ʽΪ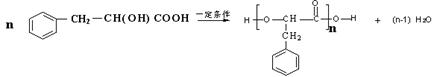
G��H����������Ӧ����ѧ����ʽΪ
��GΪ����ᣬ������������ұ���ֻ��һ��ȡ������ͬ���칹���������4�֣��ֱ���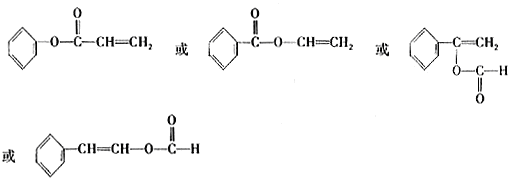
���㣺�����л������ʽ��ȷ�����ṹ��ʽ����ѧ����ʽ����д����Ӧ���͵��жϣ�ͬ���칹����ж�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ȡ9��20gֻ���ǻ����������������ŵı��Ͷ�Ԫ�������������������У�����ȼ������ȫȼ�գ�ȼ�պ�����徭��Ũ����ʱ��Ũ��������7��20g��ʣ�����徭CaO���պ��������6��72L����״���²ⶨ����
��1��9��20g����C��H��O�����ʵ����ֱ�Ϊ��C mol��H mol��O mol���ô���C��H��O��ԭ�Ӹ���֮��Ϊ ��
��2�������ϱ�ֵ�ܷ�ȷ���ô��ķ���ʽ ����ԭ���� ��
��3��������һ��̼ԭ�ӵı���һԪ���ܴ�������ȩ�����������ĸô��Ľṹ��ʽ�У� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ɽ���ۺ����̼�����ĺϳ�·�����£����Ʊ������л����Ƶø߷��Ӳ���F��
�ش��������⣺
��1����Ӧ�ٵķ�Ӧ����Ϊ ��
��2��д��D�Ľṹ���� ��
��3��E��C5H8O2�����������������ŵ����� ��
��4��д����һ��������D�� ��Ӧ�ϳɾ�̼���Ļ�ѧ����ʽ ��
��Ӧ�ϳɾ�̼���Ļ�ѧ����ʽ ��
��5��д��һ��ͬʱ��������������E��ͬ���칹��Ľṹ��ʽ ��
����E������ͬ������
���ܷ���������Ӧ
��H�˴Ź������������ֲ�ͬ��������ԭ�ӣ������ֱ�Ϊ1:2:2:3
��6��������ɫ��ѧԭ����ԭ�ӵ�������Ϊ100%���Ա�Ȳ���״�Ϊԭ�Ͽɺϳ�F������ƺϳ�·�ߣ����Լ���ѡ�� ���ϳ�·������ͼʾ�����£�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���۷��ɸĽ��л��߷��ӻ���������ʣ��߷��Ӿۺ���P�ĺϳ�·�����£�

��1��A�ĽṹʽΪ________________ ��C������Ϊ
��2��D�к��еĹ����ŵ�����
��3����F���١��ۺϳ�I��F����ʹ��ˮ��ɫ����Ӧ�ٵĻ�ѧ����ʽ�� ��
��Ӧ�ڵķ�Ӧ������ ��Ӧ�۵ķ�Ӧ�Լ��� ��
��4������˵����ȷ���� ��
a��C����ˮ����������
b��A��1,3-����ϩ��Ϊͬϵ��
c����I����Mʱ��1mol I�������3molNaOH
d��N������˳���칹��
��5��д����D����E�Ļ�ѧ����ʽ ��
��6��E��N�����ʵ����������۷�Ӧ����P����P�Ľṹ��ʽΪ ��
��7��E�ж���ͬ���칹�壬д��������������������ͬ���칹��Ľṹ��ʽ ��
a��������������ȡ����
b����ʹFeCl3Һ����ɫ
c��1mol���л�����Ũ��ˮ��Ӧʱ������4molBr2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������������������ᡢ�Ҵ��Ļ�����ͼ�Ƿ��������������ͼ���������ʵ����Լ������뷽���Լ���������й����ʵ����ơ�
(1)a________��b________��
(2)��________����________����________��
(3)A________��B_________��C________��D_______��E_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��̼������ɫ������������Ŀ�����ԣ������������Ա�����֡������ֻ�������ǵȡ�˫�ӻ������Ǻϳɾ�̼�����ĵ���֮һ��ij��˫�ӻ�����G�ĺϳ�·�����£�
��1��G�������Ĺ�������________________��B�ĺ˴Ź��������� ���塣
��2��д����Ӧ���ͣ���Ӧ �� ___________����Ӧ �� ____________��
��3��д��A������ ______________��F�Ľṹ��ʽ ______________��
��4��д����Ӧ �� �Ļ�ѧ����ʽ_______________________________________��
��5��C�ж���ͬ���칹�壬д��ͬʱ��������������ͬ���칹��Ľṹ��ʽ_____________��
��I�����ڦ�-�����ᣬ�ұ�������������Ϊ��λ��ȡ����
��II����FeCl3��Һ��������ɫ����
��III��1 mol��ͬ���칹��������NaOH��Һ��Ӧʱ�����������3 mol NaOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ�����Ǻϳ����������Ȼ�������Ҫ���裬ij�����ϳ�·�����£�
��1���������Ļ�ѧʽΪ ��
��2��������A�Ľṹ��ʽΪ ����3����Ӧ�ٵĻ�ѧ����ʽΪ ��
��4������˵��������� ��
| A����������ܷ���������Ӧ | B��������������ڷ����� |
| C����Ӧ������������Ӧ | D�������������4 molH2�����ӳɷ�Ӧ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�顣
������ʽ��ȷ����
(1)���л���A�����������г��ȼ�գ�ʵ���ã�����5.4 g H2O��8.8 g CO2����������6.72 L(��״����)����������и�Ԫ�ص�ԭ�Ӹ������� ��
(2)�����Dzⶨ���л����������Է�������Ϊ46��������ʵķ���ʽ�� ��
(3)���ݼۼ����ۣ�Ԥ��A�Ŀ��ܽṹ��д���ṹ��ʽ�� ��
���ṹʽ��ȷ����
(4)���ⶨ���л���A��������3����ԭ�ӣ���A�Ľṹ��ʽΪ ��
������ʵ�顿
(5)A��һ����������ˮ������B��B�ɺϳɰ�װ����C����д��Bת��ΪC�Ļ�ѧ��Ӧ����ʽ�� ��
(6)���������е��˶�Ա����Ť��ʱ����ҽ�漴��������(�е�12.27��)�����˲�λ���оֲ��䶳����������Bѡ����ʵķ����Ʊ������飬Ҫ��ԭ��������Ϊ100%����д���Ʊ���Ӧ�ķ���ʽ�� ��
(7)A��ͨ����ʳ��һ���������Ƶã�����ʳ�Ƶõ�A��һ���¶����ܱմ��棬��Ϊ����һϵ�еĻ�ѧ�仯����ø����㡣��д�����һ����Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ϵ�Ⱥ�����������ҵ�ʳ���Ĺ鳲;�У���һ������������Ϳ�ڵ��ϣ�Ϊ����������ʳ��·�����ַ�����и��ټ�������Ϣ�ص�һ�֡���ν��Ϣ�أ���ָͬ��������������������Ϣ�Ļ�ѧ���ʡ����ϸ�����Ϣ�ؽР�ţ����(�ֽ���Ҷ��)�����������ϻ��������ռ���������ɷ������Ϊ����C��77.86%��H��11.76%��O��10.37%����Է�������Ϊ154������Ϣ���������Ӧ�����ɳ����������ˮ��Zn��Ӧ������ˮ�⣬ʹ������˫�����ѣ������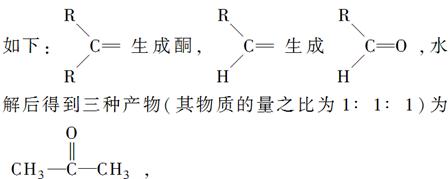
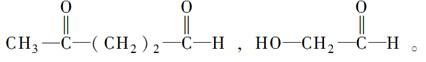
(1)ͨ������д������Ϣ�صĻ�ѧʽΪ__________________________________________��
(2)����Ϣ�صĽṹ��ʽΪ_________________________________________________��
(3)����Ϣ�ص�ϵͳ����Ϊ________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com