

 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ




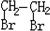
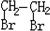





²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
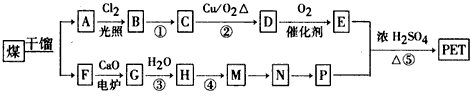
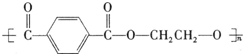 £®ÓŠČĖŅŌŹÆÓĶ²śĘ·¶Ō¶ž¼×±½ŗĶ×ī¼ņµ„µÄĻ©¾DĪŖŌĮĻ£¬Éč¼ĘŗĻ³ÉPETµÄ¹¤ŅµÉś²śĮ÷³ĢČēĻĀ£Ø·“Ó¦ÖŠ²æ·ÖĪŽ»ś·“Ó¦Īļ¼°²śĪļĪ“±ķŹ¾£©£ŗ
£®ÓŠČĖŅŌŹÆÓĶ²śĘ·¶Ō¶ž¼×±½ŗĶ×ī¼ņµ„µÄĻ©¾DĪŖŌĮĻ£¬Éč¼ĘŗĻ³ÉPETµÄ¹¤ŅµÉś²śĮ÷³ĢČēĻĀ£Ø·“Ó¦ÖŠ²æ·ÖĪŽ»ś·“Ó¦Īļ¼°²śĪļĪ“±ķŹ¾£©£ŗ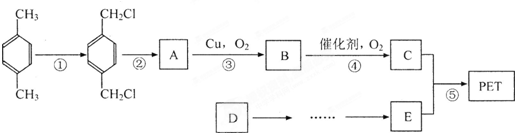
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾»ÆѧєŠŽ5£ŗÓŠ»ś»Æѧ»ł“””æ
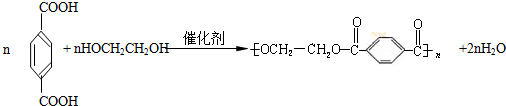
PETŹĒŹĄ½ēÉĻ²śĮæ×ī“óµÄŗĻ³ÉĻĖĪ¬£¬Ęä½į¹¹¼ņŹ½ĪŖ£ŗ
ĻÖŅŌĆŗµÄøÉĮó²śĘ·AÓėFĪŖŌĮĻÖʱøPET£¬Éś²śĮ÷³ĢČēĻĀĶ¼ĖłŹ¾”£ĘäÖŠAĪŖĢž£¬ŗ¬Ģ¼ŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ90.6%£¬ĘäÕōĘųµÄĆܶȏĒæÕĘųĆܶȵÄ3.66±¶£¬ÄÜŹ¹ĖįŠŌøßĆĢĖį¼ŲČÜŅŗĶŹÉ«£¬µ«²»ÄÜŹ¹äåĖ®ĶŹÉ«”£M·Ö×ÓĄļĖłÓŠŌ×Ó¹²Ę½Ćę”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Öø³öĻĀĮŠ·“Ó¦ĄąŠĶ£ŗ¢Ü ¢Ż
£Ø2£©Öø³ö·“Ó¦¢ŁµÄ·“Ó¦Ģõ¼ž£ŗ
£Ø3£©Š“³öÓŠ»śĪļµÄ½į¹¹¼ņŹ½£¬A£ŗ £»N£ŗ ”£
£Ø4£©Š“³öĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
·“Ó¦¢Ś ·“Ó¦¢Ū ·“Ó¦¢Ż
£Ø5£©PµÄŅ»ÖÖĶ¬ĻµĪļX·Ö×ÓŹ½ĪŖC3H8O2£¬ŌŚŗĖ“Ź²ÕńĒāĘ×Ķ¼ÖŠ³öĻÖČżÖÖŠÅŗŷ壬Ęä·åµÄĒæ¶ČÖ®±ČĪŖ2©U1©U1”£ŌņXµÄ½į¹¹¼ņŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģĮÉÄžŹ”¶«±±Óż²ÅѧŠ£ø߶žĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
PETŹĒŹĄ½ēÉĻ²śĮæ×ī“óµÄŗĻ³ÉĻĖĪ¬£¬Ęä½į¹¹¼ņŹ½ĪŖ

ŌĮĻ£¬Éč¼ĘŗĻ³ÉPETµÄ¹¤ŅµÉś²śĮ÷³ĢČēĻĀ(·“Ó¦ÖŠ²æ·ÖĪŽ»ś·“Ó¦Īļ¼°²śĪļĪ“±ķŹ¾)£ŗ

Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)·¢Éś·“Ó¦¢ŁŠč¼ÓČėµÄ·“Ó¦Īļ¼°ĘäĢõ¼žŹĒ_____________”¢_____________£»
·¢Éś·“Ó¦¢ŚµÄ·“Ó¦Īļ¼°·“Ó¦Ģõ¼žŹĒ_____________”¢_____________”£
(2)¹ż³Ģ¢ŻµÄ·“Ó¦ĄąŠĶŹĒ_____________”£
(3)Š“³ö·“Ó¦¢ŪµÄ»Æѧ·½³ĢŹ½_______________________________________”£
(4)ÓŠ»śĪļAÓŠ¶ąÖÖĶ¬·ÖŅģ¹¹Ģ壬Š“³öĀś×ćĻĀĮŠĢõ¼žµÄAµÄŅ»ÖÖĶ¬·ÖŅģ¹¹ĢåXµÄ½į¹¹¼ņŹ½£ŗ____”£
¢ŁŌŚXÖŠµĪČėFeCl3ČÜŅŗ³Ź×ĻÉ«£¬¢ŚX·Ö×Ó½į¹¹ÖŠÖ»ÓŠŅ»øö¼×»ł£¬¢Ū1molX·Ö±šÓė×ćĮæµÄ½šŹōÄĘ”¢NaOHČÜŅŗ·“Ó¦£¬ĻūŗÄn(Na)£ŗn(NaOH)=2£ŗ1”£
(5)“ÓDµ½E£¬¹¤ŅµÉĻŅ»°ćĶعż¶ž²½·“Ó¦Ą“Ķź³É”£ÓŠČĖ“ÓŌ×ÓĄūÓĆĀŹ100£„µÄ½Ē¶ČĢį³öĶعżDÓėijÖÖĪŽ»śĪļÖŹŅ»²½ŗĻ³ÉE£¬øĆĪŽ»śĪļŹĒ_____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğÉĀĪ÷Ź”øßČżµŚ11“ĪÄ£Äāæ¼ŹŌĄķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
(15·Ö)PETŹĒŹĄ½ēÉĻ²śĮæ×ī“óµÄŗĻ³ÉĻĖĪ¬£¬Ęä½į¹¹¼ņŹ½ĪŖ£ŗ
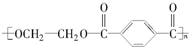
ĻÖŅŌĆŗµÄøÉĮó²śĘ·AÓėFĪŖŌĮĻÖʱøPET£¬Éś²śµÄ¹¤ŅÕĮ÷³ĢČēĶ¼ĖłŹ¾”£ĘäÖŠAĪŖĢž£¬ŗ¬Ģ¼ŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ90.6%£¬ĘäÕōĘųĆܶȏĒæÕĘųĆܶȵÄ3.66±¶£¬ĒŅÄÜŹ¹ĖįŠŌøßĆĢĖį¼ŲČÜŅŗĶŹÉ«£¬µ«²»ÄÜŹ¹äåĖ®ĶŹÉ«”£M·Ö×ÓĄļĖłÓŠŌ×Ó¹²Ę½Ćę”£
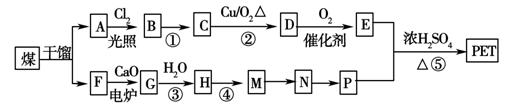
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)AµÄ·Ö×ÓŹ½ĪŖ£ŗ________£¬ĻĀĮŠ·“Ó¦ĄąŠĶĪŖ£ŗMØD”śN________£»·“Ó¦¢Ż________”£
(2)·“Ó¦¢ŁµÄ·“Ó¦Ģõ¼žĪŖ£ŗ________£»AµÄĆū³ĘĪŖ________”£
(3)Š“³öÓŠ»śĪļAĖłÓŠŅ»ĀČ“śĪļµÄ½į¹¹¼ņŹ½£ŗ
________________________________________________________________________ӣ
(4)Š“³öĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
·“Ó¦¢Ū£ŗ_______________________________________________________________£»
DÓė×ćĮæµÄĒāŃõ»ÆĶŠü×ĒŅŗÖó·Š£ŗ____________________________________________£»
(5)PµÄŅ»ÖÖĶ¬ĻµĪļXµÄ·Ö×ÓŹ½ĪŖC3H8O2£¬ŌŚŗĖ“Ź²ÕńĒāĘ×Ķ¼ÖŠ³öĻÖČżÖÖŠÅŗŷ壬Ęä·åµÄĒæ¶ČÖ®±ČĪŖ2”Ć1”Ć1”£ŌņXµÄ½į¹¹¼ņŹ½ĪŖ_________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com