



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
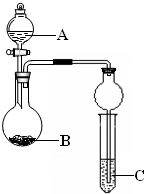
��10�֣��������й�SO2��Cl2������ʵ�顣
��1��ijС�������ͼ��ʾ��װ��ͼ��ͼ�мгֺͼ���װ����ȥ�����ֱ��о�SO2��Cl2�����ʡ�
��������˷ֱ�ͨ��SO2��Cl2��װ��A�й۲쵽�������Ƿ���ͬ�� �����ͬ������ͬ����������Dװ�������ۣ�ͨ��Cl2�����۳�ַ�Ӧʱ����Ϊ
����װ��D��װ������������������������ͨ��SO2ʱ����Kͨ������O2�Ļ�ѧ��Ӧ����ʽΪ ��
����װ��B��װ��5.0mL 1.0��10-3mol/L�ڵ�ˮ����ͨ������Cl2��ȫ��Ӧ��ת����5.0��10-5mol���ӣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��E��ʢ�� ��Һ��
��2��ijͬѧ��������SO2ͨ��һ֧װ����������Һ���Թܣ�δ���������ɣ�������Թ��м������������ĸ�� ������Կ�����ɫ����������
A����ˮ B��ϡ���� C��ϡ���� D���Ȼ���
��3������Ԫ��S��O���-2���������X��X��S��O��������Ϊ4��3����Cl2���뺬X����Һ��ȫ��Ӧ����dz��ɫ����������ȡ�ϲ���Һ�����Ȼ�����Һ���а�ɫ����������д��Cl2�뺬X����Һ��Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ������ѧ�����п��������ۺ����⣨��ѧ���֣� ���ͣ�ʵ����
��18�֣�ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
��1����ʳ�γ���������Ca2+��Mg2+��Fe3+��SO42-���������ӣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�������
����1��ȡһ�����Ĵ��Σ������ձ��У�����������ˮ����ɴ���ˮ��
����2�������ˮ�м�������Լ���Ȼ����й��ˣ���ȥ�����������Һ�м��������
����ˮ��pH��
����3�����õ�����Һ����Ũ������ȴ���ᾧ�����ˡ���ɼ��þ��Σ�
��ش��������⣺
������ʵ���еĹ��˲�����Ҫ�ձ���____________��____________�Ȳ���������
�ڲ���2�г���Na2CO3��NaOH��BaCl2��Ϊ�����Լ������������Լ���˳��Ϊ��
�� �� NaOH��
�۲���2�У��жϼ���BaCl2�ѹ����ķ�����
________________________��
��2��Ϊ���龫�δ��ȣ�������100 mL 0��5mol/L�����Σ���Һ����ͼ�Ǹ�
ͬѧת����Һ��ʾ��ͼ��ͼ�еĴ�����

________________________________________________��
���ڶ���ʱ���ӣ���������Һ��Ũ��_______0��5mol/L�������
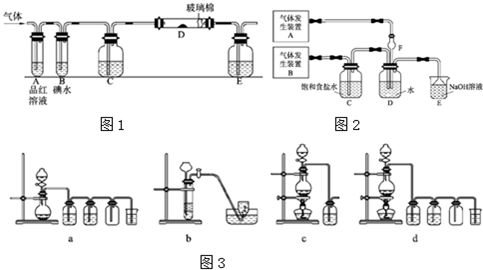
��3����ⱥ��ʳ��ˮ��װ����ͼ��ʾ�����ռ���H2Ϊ2L��
����ͬ�������ռ���Cl2 ���������
2L��ԭ��
��4��ijѧϰС�����������ͼʵ�飬����������ͨ������װ
������֤���������ʣ�
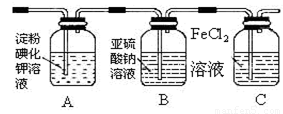
��ͨ��������A�е������� ������ʵ��װ�ô��ڵ�����ȱ
������������������������������������������������������������
��Cװ���з�����Ӧ�����ӷ���ʽΪ ��
��������С��ͬѧ���һ��ʵ�飬֤��ϴ��ƿB�е�Na2SO3�ѱ�����������ʵ�鲽�裩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ����11���¿���ѧ���Ծ� ���ͣ�ʵ����
��10�֣��������й�SO2��Cl2������ʵ�顣
��1��ijС�������ͼ��ʾ��װ��ͼ��ͼ�мгֺͼ���װ����ȥ�����ֱ��о�SO2��Cl2�����ʡ�
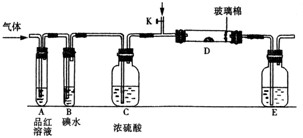
��������˷ֱ�ͨ��SO2��Cl2��װ��A�й۲쵽�������Ƿ���ͬ�� �����ͬ������ͬ����������Dװ�������ۣ�ͨ��Cl2�����۳�ַ�Ӧʱ����Ϊ
����װ��D��װ������������������������ͨ��SO2ʱ����Kͨ������O2�Ļ�ѧ��Ӧ����ʽΪ ��
����װ��B��װ��5.0 mL 1.0��10-3mol/L�ڵ�ˮ����ͨ������Cl2��ȫ��Ӧ��ת����5.0��10-5mol���ӣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��E��ʢ�� ��Һ��
��2��ijͬѧ��������SO2ͨ��һ֧װ����������Һ���Թܣ�δ���������ɣ�������Թ��м������������ĸ�� ������Կ�����ɫ����������
A����ˮ B��ϡ���� C��ϡ���� D���Ȼ���
��3������Ԫ��S��O���-2���������X��X��S��O��������Ϊ4��3����Cl2���뺬X����Һ��ȫ��Ӧ����dz��ɫ����������ȡ�ϲ���Һ�����Ȼ�����Һ���а�ɫ����������д��Cl2�뺬X����Һ��Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������й�SO2��Cl2������ʵ�顣
��1��ijС�������ͼ��ʾ��װ��ͼ��ͼ�мгֺͼ���װ����ȥ�����ֱ��о�SO2��Cl2�����ʡ�
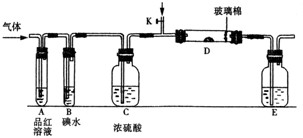
��������˷ֱ�ͨ��SO2��Cl2��װ��A�й۲쵽�������Ƿ���ͬ�� �����ͬ������ͬ����������Dװ�������ۣ�ͨ��Cl2�����۳�ַ�Ӧʱ����Ϊ
����װ��D��װ������������������������ͨ��SO2ʱ����Kͨ������O2�Ļ�ѧ��Ӧ����ʽΪ ��
����װ��B��װ��5.0 mL 1.0��10-3mol/L�ڵ�ˮ����ͨ������Cl2��ȫ��Ӧ��ת����5.0��10-5mol���ӣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��E��ʢ�� ��Һ��
��2��ijͬѧ��������SO2ͨ��һ֧װ����������Һ���Թܣ�δ���������ɣ�������Թ��м������������ĸ�� ������Կ�����ɫ����������
A����ˮ B��ϡ���� C��ϡ���� D���Ȼ���
��3������Ԫ��S��O���-2���������X��X��S��O��������Ϊ4��3����Cl2���뺬X����Һ��ȫ��Ӧ����dz��ɫ����������ȡ�ϲ���Һ�����Ȼ�����Һ���а�ɫ����������д��Cl2�뺬X����Һ��Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com