
���� ��1������Ϊǿ�������ȫ���룬�õȺţ�
��2������Ϊǿ�������ȫ���룬�õȺţ�
��3��������Ϊǿ����ʣ���ȫ���룬�õȺţ�
��4��̼�����Ϊǿ����ʣ���ȫ���룬�õȺţ�
��5���������Ϊǿ����ʣ���ȫ���룬�õȺţ�
��6�������Ϊǿ����ʣ���ȫ���룬�õȺţ�
��7��Fe��CuSO4��Һ��Ӧ��������������ͭ��
��8�����߷�Ӧ���������ƺ�ˮ��
��9��NaCl��Һ��AgNO3��Һ��Ӧ���������ƺ��Ȼ�����
��10��Ba��OH��2��Һ��H2SO4��Һ��Ӧ�������ᱵ��ˮ��
��� �⣺��1������Ϊǿ�������ȫ���룬�õȺţ����뷽��ʽ��H2SO4�T2H++SO42-��
�ʴ�Ϊ��H2SO4�T2H++SO42-��
��2������Ϊǿ�������ȫ���룬�õȺţ����뷽��ʽ��Ba��OH��2�TBa2++2OH-��
�ʴ�Ϊ��Ba��OH��2�TBa2++2OH-��
��3��������Ϊǿ����ʣ���ȫ���룬�õȺţ����뷽��ʽ��CH3COONa�TNa++CH3COO-��
�ʴ�Ϊ��CH3COONa�TNa++CH3COO-��
��4��̼�����Ϊǿ����ʣ���ȫ���룬�õȺţ����뷽��ʽ��KHCO3�TK++HCO3-��
�ʴ�Ϊ��KHCO3�TK++HCO3-��
��5���������Ϊǿ����ʣ���ȫ���룬�õȺţ����뷽��ʽ��NaHSO4�TNa++H++SO42-��
�ʴ�Ϊ��NaHSO4�TNa++H++SO42-��
��6�������Ϊǿ����ʣ���ȫ���룬�õȺţ����ӷ���ʽ��NH4NO3�TNH4++NO3-��
�ʴ�Ϊ��NH4NO3�TNH4++NO3-��
��7��Fe��CuSO4��Һ��Ӧ��������������ͭ�����ӷ���ʽ��Fe+Cu2+�TFe2++Cu��
�ʴ�Ϊ��Fe+Cu2+�TFe2++Cu��
��8�����߷�Ӧ���������ƺ�ˮ�����ӷ���ʽ��H++OH-�TH2O��
�ʴ�Ϊ��H++OH-�TH2O��
��9��NaCl��Һ��AgNO3��Һ��Ӧ���������ƺ��Ȼ��������ӷ���ʽ��Cl-+Ag+�TAgCl����
�ʴ�Ϊ��Cl-+Ag+�TAgCl����
��10��Ba��OH��2��Һ��H2SO4��Һ��Ӧ�������ᱵ��ˮ�����ӷ���ʽ��Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O��
�ʴ�Ϊ��Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O��
���� ���⿼�������ӷ���ʽ�����뷽��ʽ����д����ȷ�����ǿ�������뷽ʽ����Ϥ���ӷ�Ӧʵ���ǽ���ؼ�����Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �ۺ�����CO2��CO�Թ�����̼�������Ҫ���壮
�ۺ�����CO2��CO�Թ�����̼�������Ҫ���壮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | KԽ��Ӧ�̶�Խ�� | B�� | KԽ��Ӧ�̶�ԽС | ||
| C�� | K�Ĵ�С�뷴Ӧ�̶��� | D�� | �����¶ȣ�K���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
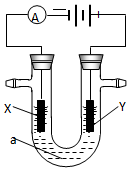 �� 1 ����ҵ��ͨ����ⱥ���Ȼ�����Һ�ķ�������������ƣ��ҹ����ȼҵ���������ӽ���Ĥ���ۣ�д����ⱥ���Ȼ�����Һʱ�ĵ缫��Ӧ���ܵ����ӷ�Ӧ����ʽ��
�� 1 ����ҵ��ͨ����ⱥ���Ȼ�����Һ�ķ�������������ƣ��ҹ����ȼҵ���������ӽ���Ĥ���ۣ�д����ⱥ���Ȼ�����Һʱ�ĵ缫��Ӧ���ܵ����ӷ�Ӧ����ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
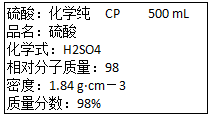 ��ͼ�������Լ�ƿ��ǩ�ϵ����ݣ�
��ͼ�������Լ�ƿ��ǩ�ϵ����ݣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ά��������ϳ������أ�ͻ�����������л���Ľ��� | |
| B�� | �������ܡ�̫���ܵ�����Դ���ƹ��Ҵ����ͣ�ʹ������ϴ�Ӽ�����ֱ�ӽ���̼�ŷ��� | |
| C�� | ��������ǡ��˴Ź����ǡ������Ƕ��������л�������ṹ�ķ��� | |
| D�� | ��������Ҫ�Ļ���ԭ�ϣ���ֽ��������ںϳɰ�����ҵ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na+��K+��SO42-��CO32- | B�� | Cu2+��K+��SO42-��NO3- | ||
| C�� | Na+��K+��Cl-��NO3- | D�� | Mg2+��K+��HCO3-��Cl- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com