
| �� |
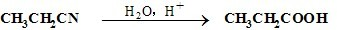
| Br2��P |
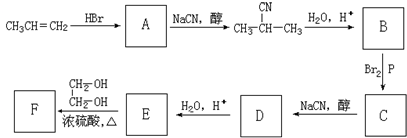
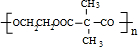 �����F����ʽ����ȷ����ṹ��C7H12O5�Ľṹ��ʽΪ
�����F����ʽ����ȷ����ṹ��C7H12O5�Ľṹ��ʽΪ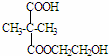 ����Ԫ��״��Ϊ
����Ԫ��״��Ϊ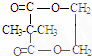 ���ݴ˷������
���ݴ˷������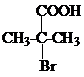 ����Ϸ�Ӧ��Ϣ��֪��DΪ
����Ϸ�Ӧ��Ϣ��֪��DΪ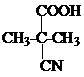 ��EΪ��CH3��2C ��COOH��2��E���Ҷ�����Ӧʱ�ɷ���������Ӧ��������Ӧ�ֱ��γ���״������״���߾���γɸ߾���ʱF�Ľṹ��ʽΪ
��EΪ��CH3��2C ��COOH��2��E���Ҷ�����Ӧʱ�ɷ���������Ӧ��������Ӧ�ֱ��γ���״������״���߾���γɸ߾���ʱF�Ľṹ��ʽΪ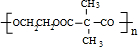 �����F����ʽ����ȷ����ṹ��C7H12O5�Ľṹ��ʽΪ
�����F����ʽ����ȷ����ṹ��C7H12O5�Ľṹ��ʽΪ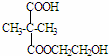 ����Ԫ��״��Ϊ
����Ԫ��״��Ϊ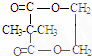 ��
��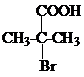 +NaCN
+NaCN | �� |
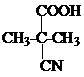 ��
��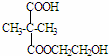 ���ʴ�Ϊ��
���ʴ�Ϊ��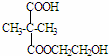 ��
��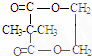 ���ʴ�Ϊ��
���ʴ�Ϊ��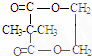 ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
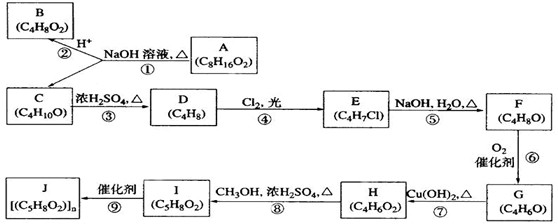
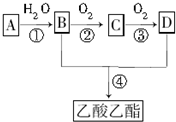 ������ʯ�ͺ�ú�����ֻ�������ԭ�ϡ�A�ͼף�A����̬��������Һ̬����B��D�����������ֳ������л����AΪ��Ҫԭ�Ϻϳ�������������ϳ�·����ͼ��ʾ��
������ʯ�ͺ�ú�����ֻ�������ԭ�ϡ�A�ͼף�A����̬��������Һ̬����B��D�����������ֳ������л����AΪ��Ҫԭ�Ϻϳ�������������ϳ�·����ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
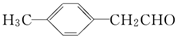 ��ת��Ϊ�Լ�����Ȳ��
��ת��Ϊ�Լ�����Ȳ��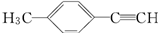 ����һ���ϳ�·�����£�
����һ���ϳ�·�����£�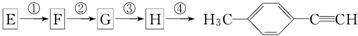 ��GΪ��Է�������Ϊ118��������
��GΪ��Է�������Ϊ118��������| ��� | �����Լ�����Ӧ���� | ��Ӧ���� |
| �� | ||
| �� | ||
| �� | ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
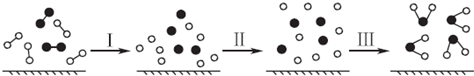
| A���ù��̱�ʾ�ķ�ӦΪ��2H2+O2�T2H20 |
| B�����̢������ȹ��̣����̢��Ƿ��ȹ��� |
| C�����̢��л�ѧ������ |
| D���÷�Ӧ�������仯��һ����������ʽ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��x������Ԫ����� |
| B��xֻ�л�ԭ�� |
| C��x�к�̼40% |
| D��x�ķ������м� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��26.3% | B��73.7% |
| C��67.7% | D��95% |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com