
(14分)正丁醚(CH3CH2CH2CH2OCH2CH2CH2CH3)是一种化工原料,常温下为无色液体,不溶于水,沸点为142.4℃,密度比水小。某实验小组利用如下装置合成正丁醚(其它装置均略去),发生的主要反应为:2CH3CH2CH2CH2OH CH3CH2CH2CH2OCH2CH2CH2CH3+H2O
CH3CH2CH2CH2OCH2CH2CH2CH3+H2O
实验过程如下:在容积为100mL的三颈烧瓶中将5mL浓硫酸、14.8g正丁醇和几粒沸石混合均匀,再加热回流一段时间,收集到粗产品,精制得到正丁醚。回答下列问题:
(1)合成粗产品时,液体试剂加入顺序是 。
(2)实验中冷凝水应从 口出去(填“a”或“b”)。
(3)为保证反应温度恒定在135℃,装置C中所盛液体必须具有的物理性质为 。
(4)加热时间过长或温度过高,反应混合液会变黑,写出用NaOH溶液吸收有毒尾气的离子方程式 。
(5)得到的正丁醚粗产品依次用8 mL50%的硫酸、10 mL水萃取洗涤。该步骤中需要的属于硅酸盐材质的实验仪器是烧杯、玻璃棒、 。该仪器使用前需要 。
(6)将分离出的有机层用无水氯化钙干燥,过滤后再进行 (填操作名称)精制得到正丁醚。
(7)本实验最终得到6.50g正丁醚,则正丁醚的产率是 。
(1)先加正丁醇,后加浓硫酸;(2)a;(3)该液体沸点大于135℃;
(4)2OH-+SO2=SO32-+H2O;(5)分液漏斗; 检漏;(6)蒸馏;(7)50.0%。
解析试题分析:(1)合成粗产品时,由于丁醇的密度比硫酸小,而且二者混合会放出大量的热,所以液体试剂加入顺序是先加正丁醇,后加浓硫酸;(2)为了使冷凝效果更好,一个使冷凝管内充满水,所以实验中冷凝水应从下口b加入,从上口a流出;(3)为保证反应温度恒定在135℃,装置C中所盛液体必须具有的物理性质为该液体沸点大于135℃;(4)加热时间过长或温度过高,会发生负反应产生SO2、CO2、C单质等;反应混合液会变黑,写出用NaOH溶液吸收有毒尾气的离子方程式是2OH-+SO2=SO32-+H2O;(5)得到的正丁醚粗产品依次用8 mL50%的硫酸、10 mL水萃取洗涤。该步骤中需要的属于硅酸盐材质的实验仪器是烧杯、玻璃棒、)分液漏斗;该仪器使用前需要检查是否漏液;(6)将分离出的有机层用无水氯化钙干燥,过滤后再进行蒸馏,精制得到正丁醚。(7)14.8g正丁醇的物质的量是14.8g÷74g/mol=0.2mol,根据反应方程式可知若完全反应,则应该生成0.1mol的正丁醚,因为正丁醚的相对分子质量是130,所以0.1mol的正丁醚的质量是13g,而本实验最终得到6.50g正丁醚,则正丁醚的产率是(6.50g ÷13g )×100%=50.0%。
考点:考查物质的制取,作用包括物质的混合、操作过程、仪器的选择与使用、混合物的分离、产率的计算的知识。


 天天向上一本好卷系列答案
天天向上一本好卷系列答案 小学生10分钟应用题系列答案
小学生10分钟应用题系列答案科目:高中化学 来源: 题型:单选题
下图所示是一套实验室制气装置,用于发生、干燥和收集气体。下列各组物质中能利用这套装置进行实验的是 
| A.铜屑和浓硝酸 | B.二氧化锰和浓盐酸 |
| C.氯酸钾和MnO2 | D.碳酸钙和稀盐酸 |
查看答案和解析>>
科目:高中化学 来源: 题型:填空题
纳米氧化镁具有特殊的热、光、电、力学和化学等性能,有广泛的应用前景。下图是利用海水制盐的副产品制备纳米氧化镁的流程图。
(1)操作I包括蒸发结晶、____________。
(2)操作I后得到的母液中镁离子浓度为1.8×10-3 mol·L-1,要使镁离子产生沉淀,溶液的pH最低应为_____________。(已知:Ksp[Mg(OH)2]= 1.8×10-11)
(3)反应I中CO(NH2)2与H2O反应生成CO2和NH3·H2O,还发生另一主要化学反应的离子方程式为______________________________。
(4)某科研小组研究反应I在378K~398K时的反应时问、反应物的物质的量配比等因素对制备纳米氧化镁产率的影响。请完成以下实验设计表:
| 实验 编号 | T/K | 反应 时间/h | 反应物的物质的量配比 n[CO(NH2)2]∶n[MgCl2?6H2O] | 实验目的 |
| ① | 378 | 3 | 3∶1 | (I)实验①和③探究探究反应物的物质的量配比对产率的影响; (II)实验②和④探究 ; (III)实验②和 探究反应时间对产 率的影响。 |
| ② | 378 | 4 | 4∶1 | |
| ③ | 378 | 3 | | |
| ④ | 398 | 4 | 4∶1 |

查看答案和解析>>
科目:高中化学 来源: 题型:问答题
(15分)用磷灰石制磷肥的副产品六氟硅酸钠(Na2SiF6)可制冰晶石(Na3AlF6),冰晶石是电解铝的助熔剂,可降低氧化铝的熔点。下图是工业制铝的流程图: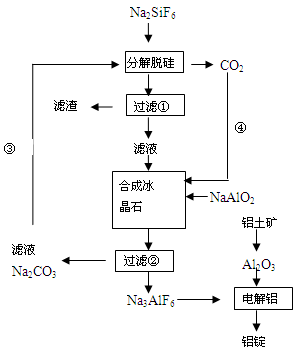
(1)工业上从铝土矿制备较高纯度Al2O3的主要工艺流程需__________个环节,第一步反应的方程式______________________________________________________________
(2)该制备工艺中有两次过滤操作,过滤操作①的滤液是________溶液,滤渣是________ 。
(3)分解脱硅和合成冰晶石化学反应方程式分别为:_________________、____________________。
(4)工艺过程中③和④的目的是_____________________,碳酸钠和二氧化碳是否够用 。
(5)电解Al2O3制Al时,I=200kA,一天制Al 1.430 t,电解效率是多少?
查看答案和解析>>
科目:高中化学 来源: 题型:实验题
下图中的实验装置可以用于实验室制取乙炔。请填空:
(1)图中A管的作用是______________,制取乙炔的化学反应方程式为____________________________。
(2)乙炔通入酸性KMnO4溶液中,发生___________反泣,可以观察到__________________。现象,若通入溴水中,发生______________反应。
(3)乙炔燃烧时的现象为____________________,为了安全,点燃乙炔前应先______________。
查看答案和解析>>
科目:高中化学 来源: 题型:实验题
(12分)SnSO4是一种重要的硫酸盐,在工业生产中有着广泛的应用。其制备路线如下: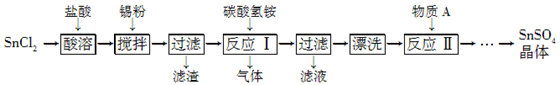
已知:在酸性条件下,溶液中的Sn2+可被空气中的氧气氧化成Sn4+; SnCl2能水解生成碱式氯化亚锡[Sn(OH)Cl]。
(1) 写出物质A的名称:________。
(2) SnCl2用盐酸而不用水溶解的原因是____________________(用化学方程式表示)。
(3) 锡粉的作用是除去酸溶时产生的少量Sn4+,请写出产生Sn4+的离子方程式:______________________________。
(4) 反应Ⅰ生成的沉淀为SnO,写出该反应的化学方程式:____。该反应的温度需要控制在75 ℃左右的原因是____。
(5) 实验室中“漂洗”沉淀的实验操作方法是____。
查看答案和解析>>
科目:高中化学 来源: 题型:实验题
三氯化六氨合钴(Ⅲ)是一种重要的化工产品, 实验中采用H2O2作氧化剂,在大量氨和氯化铵存在下,选择活性炭作为催化剂将Co(Ⅱ)氧化为Co(Ⅲ),来制备三氯化六氨合钴(Ⅲ)配合物,反应式为:2CoCl2·6H2O + 10NH3 + 2NH4Cl + H2O2 活性炭2[Co(NH3)6]Cl3 +14H2O
已知:① 钴(Ⅱ)与氯化铵和氨水作用,经氧化后一般可生成三种产物:紫红色的二氯化一氯五氨合钴[Co(NH3)5 Cl]Cl2晶体、砖红色的三氯化五氨一水合钴[Co(NH3)5 H2O]Cl3晶体、橙黄色的三氯化六氨合钴[Co(NH3)6]Cl3晶体,控制不同的条件可得不同的产物(如温度不同产物也不同)。293K时,[Co(NH3)6]Cl3在水中的溶解度为0.26mol/L
②CoCl2、[Co(NH3)6]Cl3、[Co(NH3)5 Cl]Cl2在水中能完全电离
③制备三氯化六氨合钴(Ⅲ)粗产品的流程如下:
④三氯化六氨合钴(Ⅲ)粗产品的提纯流程如下:
⑤制备过程中可能用到的部分装置如下:
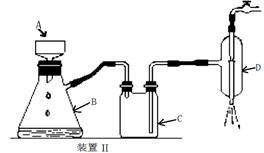

请回答下列问题:
(1)操作B的名称是 ,操作A后所弃去的沉淀中一定有 。
(2)CoCl2在没有铵盐存在的情况下,遇氨水生成蓝色沉淀,该反应的离子方程式为 。原料NH4Cl的主要作用有:① ;②提供NH3。
(3)冷却至10℃后,步骤B中逐滴加入H2O2溶液的目的:① ;②使反应温和进行。
(4)操作A的名称为 ,进行该操作的装置是 (填序号)。
A.装置Ⅰ B.装置Ⅱ C.装置Ⅲ
若操作过程中,发现漏斗尖嘴处有少量晶体析出,处理方法是 。
装置Ⅱ中仪器A、B、C、D的名称分别是 、 、 、 。
(5)步骤C进行洗涤时要用到两种试剂,应该先用_____(填序号,下同)洗涤,后用 洗涤。
A. 饱和氯化钠溶液 B. 无水乙醇 C. 浓盐酸
(6)你认为本实验提高产率的关键步骤有哪些? 。
(7)与[Co(NH3)6]Cl3类似的产品还有[Co(NH3)5Cl]Cl2,请简述验证某晶体是[Co(NH3)6]Cl3还是
[Co(NH3)5Cl]Cl2的实验方案: 。
查看答案和解析>>
科目:高中化学 来源: 题型:实验题
(14分)某课外学习小组为探究硫酸亚铁晶体(FeSO4·7H2O)制备及影响因素,进行如下实验。
Ⅰ制取硫酸亚铁溶液
称取一定量铁屑,放入烧瓶中,加入25 mL 3 mol·L-1硫酸,用酒精灯加热。
(1)加热一段时间后,发现烧瓶中溶液变黄并产生能使品红溶液褪色的气体。产生该现象的原因是
(用化学方程式表示)。
(2)制备过程中,铁需过量的原因是 。
Ⅱ实验改进
该小组同学发现以上实验不完善,查阅资料后,对实验做如下
改进:①反应前通入氮气;②水浴加热,控制温度50~60℃;
③实验中适当补充硫酸调节pH=2;④增加尾气吸收装置。改进装置如图(夹持和加热装置略去)。
(3)实验中采用水浴加热,除了便于控制温度外,还有的优点是 。
(4)调节pH=2目的是 。
(5)下列装置适合尾气吸收的是 (填序号)。
Ⅲ 晶体纯度测定
(6)烧瓶中的溶液经处理得硫酸亚铁晶体。准确称取晶体 0.5000 g置于锥形瓶中,加入10 mL 3mol·L-1硫酸溶液和15 mL新煮沸过的蒸馏水进行溶解,立即用0.02000mol·L-1高锰酸钾标准溶液滴定,消耗标准溶液体积的平均值为16.90 mL。(已知:5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O)
①高锰酸钾标准溶液应盛装在 滴定管(填“酸式”或“碱式”)。
②获得硫酸亚铁晶体的纯度为 。
查看答案和解析>>
科目:高中化学 来源: 题型:实验题
碳铵是一种较常使用的化肥,它在常温下易分解。某化学兴趣小组对碳铵的成分存在疑问,进行了如下探究。
【定性实验】检验溶液中的阴、阳离子。
取少量固体放入试管中,加入盐酸,把生成的气体通入澄清石灰水中,有白色沉淀生成。再另取少量碳铵放入试管中,加入浓NaOH溶液,加热,用湿润的红色石蕊试纸检验生成的气体,石蕊试纸变蓝色。
(1)根据实验现象,推测碳铵中所含有阴离子可能是 和 。
(2)据实验现象,碳铵与足量NaOH溶液加热反应的离子方程式可能是 。
【定量实验】测定碳铵中C元素和N元素质量比。
该兴趣小组准确称取ag碳铵,加热使之分解,并把产物通入碱石灰中,如下图所示。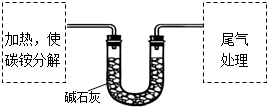
(1)碳铵固体应放在 中进行加热。
| A.试管 | B.蒸发皿 | C.烧瓶 | D.坩埚 |
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com