
 ��֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������A��B��C��ͬһ���ڵķǽ���Ԫ�أ�������DCΪ���ӻ����D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ���������壬ͨ�����ʯ��ˮ�У�ʯ��ˮ����ǣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ�EԪ���ڵؿ��к���λ�ӵ���λ��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����磮���������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
��֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������A��B��C��ͬһ���ڵķǽ���Ԫ�أ�������DCΪ���ӻ����D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ���������壬ͨ�����ʯ��ˮ�У�ʯ��ˮ����ǣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ�EԪ���ڵؿ��к���λ�ӵ���λ��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����磮���������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ������ A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ�����֮����������ΪN��O��F�е����֣�A��B��C��ͬһ���ڵķǽ���Ԫ�أ����ڵڶ����ڣ�AC2Ϊ���������壬ͨ�����ʯ��ˮ�У�ʯ��ˮ����ǣ�������Ϊ������̼������֪AΪ̼Ԫ�ء�CΪ��Ԫ�أ�Bԭ����������̼����֮�䣬��BΪNԪ�أ�D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��C�γ�-2�������ӣ����������Ϊ10����Dԭ�Ӻ��������Ϊ10+2=12����DΪMg��EԪ���ڵؿ��к���λ�ӵ���λ����EΪFeԪ�أ�FeCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Fe��NH3��4��H2O��2]Cl3���ݴ˽��
��� �⣺A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ�����֮����������ΪN��O��F�е����֣�A��B��C��ͬһ���ڵķǽ���Ԫ�أ����ڵڶ����ڣ�AC2Ϊ���������壬ͨ�����ʯ��ˮ�У�ʯ��ˮ����ǣ�������Ϊ������̼������֪AΪ̼Ԫ�ء�CΪ��Ԫ�أ�Bԭ����������̼����֮�䣬��BΪNԪ�أ�D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��C�γ�-2�������ӣ����������Ϊ10����Dԭ�Ӻ��������Ϊ10+2=12����DΪMg��EԪ���ڵؿ��к���λ�ӵ���λ����EΪFeԪ�أ�FeCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Fe��NH3��4��H2O��2]Cl3��
��1��C���⻯��ΪH2O��������Oԭ�ӳ�2���Ҽ�������2�Թ¶Ե��ӣ����ӵ����幹����V�Σ�����Oԭ�Ӳ�ȡsp3 �ӻ����ʴ�Ϊ��V�Σ�sp3��
��2��EΪFeԪ�أ�ԭ������Ϊ26��ԭ�Ӻ�����26�����ӣ���������Ų�ʽ�� 1s22s22p63s23p63d64s2��FeCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Fe��NH3��4��H2O��2]Cl3��
�ʴ�Ϊ��1s22s22p63s23p63d64s2��[Fe��NH3��4��H2O��2]Cl3��
��3��ͬ������ԭ����������һ�����ܳ��������ƣ�NԪ��2p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ����������˼�ǣ��ʵ�һ��������С�����˳��ΪC��O��N���ʴ�Ϊ��C��O��N��
��4��������AC2ΪCO2��һ����N��O��ɵĻ�������CO2��Ϊ�ȵ����壬�仯ѧʽΪ N2O���ʴ�Ϊ��N2O��
��5��ͼ1�ṹ��Ԫ��ԭ����ĿΪ1+8��$\frac{1}{8}$=2��ͼ2�ṹ��Ԫ��ԭ����ĿΪ6��$\frac{1}{2}$+8��$\frac{1}{8}$=4�����߽ṹ��Ԫ�к���ԭ����Ŀ֮��2��4=1��2���ʴ�Ϊ��1��2��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��漰��������Ų������ӽṹ���ӻ���������������ܡ���������ȣ��⻯��ķе������ͬ����������Ԫ���⻯��ķе�����ƶϵ�ͻ�ƿڣ��Ѷ��еȣ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2Na+2NH3��2NaNH2+H2�� | B�� | NH3+HNO3��NH4NO3 | ||
| C�� | 4NH3+6NO��5N2+6H2O | D�� | 3SiH4+4NH3��Si3N4+12H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ƹ�ʹ��ȼú������ | B�� | ʵʩ�̻����� | ||
| C�� | ���ƿ���ȼ�ϵ������ | D�� | ������չ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | +$\frac{aM}{11.2m}$ | B�� | +$\frac{am}{11.2M}$ | C�� | +$\frac{11��2m}{aM}$ | D�� | +$\frac{aM}{22.4m}$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 4 | B�� | 3 | C�� | 2 | D�� | 1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����HNO3�ữ��Ba��NO3��2��Һ | |
| B�� | �ȼ���HNO3�ữ���ټ�Ba��NO3��2 | |
| C�� | ���������ữ��BaCl2 | |
| D�� | ���������ữ�����г���������ˣ���Һ���ټ�BaCl2��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ��������ζ | B�� | 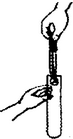 ȡ�ÿ�״���� | ||
| C�� | 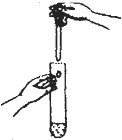 �μ�Һ�� | D�� | 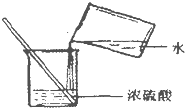 ϡ��Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3CH2 CH2OH | B�� | CH3COOH | C�� | H2O | D�� | H2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
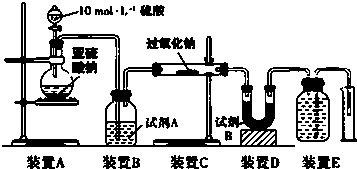
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com