
��10�֣��������п�ͼ�ش�����
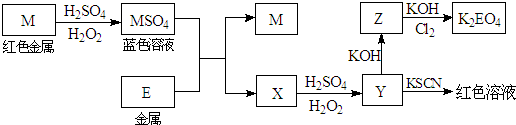
������ʱ������ʽ�е�M��E������Ӧ��Ԫ�ط��ű�ʾ����

��1��д��M����ϡH2SO4��H2O2���Һ�����ӷ���ʽ�� ��
��2��ijͬѧȡX����Һ����ϡ�����ữ������������KI��Һ����Һ��Ϊ��ɫ��д���������仯������ص����ӷ���ʽ�� �� ��
��3��д��Cl2��Z����ΪK2EO4�Ļ�ѧ����ʽ�� ��
��4����E�Ʊ�����E��C2H5��2�Ľṹ����ͼ��������ԭ�ӵĻ�ѧ������ȫ��ͬ������������ȴ�������Ϊ���ĽṹΪ�� ���˴Ź����ܹ����������ֽṹ���ں˴Ź��������У���ȷ�Ľṹ�� �ַ壬����Ľṹ��
�ַ塣
���˴Ź����ܹ����������ֽṹ���ں˴Ź��������У���ȷ�Ľṹ�� �ַ壬����Ľṹ��
�ַ塣


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2008?���գ��������п�ͼ�ش����⣨����ʱ������ʽ�е�M��E������Ӧ��Ԫ�ط��ű�ʾ����
��2008?���գ��������п�ͼ�ش����⣨����ʱ������ʽ�е�M��E������Ӧ��Ԫ�ط��ű�ʾ����
 ���˴Ź����ܹ����������ֽṹ���ں˴Ź��������У���ȷ�Ľṹ��
���˴Ź����ܹ����������ֽṹ���ں˴Ź��������У���ȷ�Ľṹ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com