
(7·Ö)ijĮņĖį³§ŌŚ½ųŠŠ»ĘĢśæó(Ö÷ŅŖ³É·ÖŹĒFeS2)³É·Ö²ā¶ØŹ±£¬Č”0£®1000 gѳʷŌŚæÕĘųÖŠ³ä·Ö×ĘÉÕ£¬½«Éś³ÉµÄSO2ĘųĢåÓė×ćĮæFe2(SO4)3ČÜŅŗĶźČ«·“Ó¦ŗó£¬ÓĆÅضČĪŖ0£®02000 mol£ÆLµÄK2Cr2O7±ź×¼ČÜŅŗµĪ¶ØÖĮÖÕµć£¬ĻūŗÄK2Cr2O7ČÜŅŗ25£®00 mL”£
ŅŃÖŖ£ŗ¢Ł![]()
¢Ś ![]() +
+ ![]()
![]()
![]() +
+ ![]() + +H2O
+ +H2O
(1)ŌŚ·“Ó¦¢ŚµÄŗįĻßÉĻĢīŠ“ĻąÓ¦µÄ»Æѧ¼ĘĮæŹż”£
(2)Čō×ĘÉÕ6 gFeS2²śÉśµÄSO2Č«²æ×Ŗ»ÆĪŖSO3ĘųĢåŹ±·Å³ö9£®83 kJČČĮ棬Š“³öSO2ĘųĢå×Ŗ»ÆĪŖSO3µÄČČ»Æѧ·½³ĢŹ½£ŗ ”£
(3)Ēóѳʷ֊FeS2µÄÖŹĮæ·ÖŹż(¼ŁÉčŌÓÖŹ²»²Ī¼Ó·“Ó¦)£¬Š“³ö¼ĘĖć¹ż³Ģ”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
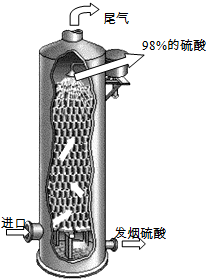 £Ø2011?Ģ©ÖŻ¶žÄ££©ĮņĖįŌŚ¹śĆń¾¼ĆÖŠÕ¼ÓŠ¼«ĘäÖŲŅŖµÄµŲĪ»£¬ĪŅ¹ś³£ŅŌ»ĘĢśæó£ØÖ÷ŅŖ³É·ÖFeS2£©ĪŖŌĮĻÉś²śĮņĖį£®
£Ø2011?Ģ©ÖŻ¶žÄ££©ĮņĖįŌŚ¹śĆń¾¼ĆÖŠÕ¼ÓŠ¼«ĘäÖŲŅŖµÄµŲĪ»£¬ĪŅ¹ś³£ŅŌ»ĘĢśæó£ØÖ÷ŅŖ³É·ÖFeS2£©ĪŖŌĮĻÉś²śĮņĖį£®| ³É·Ö | “ß»ÆŃõ»ÆĒ° | “ß»ÆŃõ»Æŗó |
| O2 | 11% | Ī“²ā¶Ø |
| SO2 | 7% | Ī“²ā¶Ø |
| N2 | 82% | 84.8% |
| SO3 | -- | 6.9% |
| 800Q |
| a |
| 800Q |
| a |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
2- 7 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015½ģÉĻŗ£ŹŠø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
»ĘĢśæóÖ÷ŅŖ³É·ÖŹĒFeS2”£Ä³ĮņĖį³§ŌŚ½ųŠŠ»ĘĢśæó³É·Ö²ā¶ØŹ±£¬Č”0.1000 gѳʷŌŚæÕĘųÖŠ³ä·Ö×ĘÉÕ£¬½«Éś³ÉµÄSO2ĘųĢåÓė×ćĮæFe2(SO4)3ČÜŅŗĶźČ«·“Ó¦ŗó£¬ÓĆÅضČĪŖ0.02000 mol£ÆLµÄK2Cr2O7±ź×¼ČÜŅŗµĪ¶ØÖĮÖÕµć£¬ĻūŗÄK2Cr2O7ČÜŅŗ25.00 mL ”£
ŅŃÖŖ£ŗSO2 + 2Fe3+ + 2H2O”śSO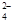 + 2Fe2+ + 4H+
+ 2Fe2+ + 4H+
Cr2O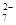 + 6 Fe2+ + 14 H+
”ś2 Cr3+ + 6 Fe3+ + 7 H2O
+ 6 Fe2+ + 14 H+
”ś2 Cr3+ + 6 Fe3+ + 7 H2O
£Ø1£©ŃłĘ·ÖŠFeS2µÄÖŹĮæ·ÖŹżŹĒ£Ø¼ŁÉčŌÓÖŹ²»²Ī¼Ó·“Ó¦£© ”£
£Ø2£©Čō×ĘÉÕ6 g FeS2²śÉśµÄSO2Č«²æ×Ŗ»ÆĪŖSO3ĘųĢåŹ±·Å³ö9.83 kJČČĮ棬²śÉśµÄSO3ÓėĖ®Č«²æ»ÆŗĻÉś³ÉH2SO4£¬·Å³ö13.03kJČČĮ棬Š“³öSO3ĘųĢå×Ŗ»ÆĪŖH2SO4µÄČČ»Æѧ·½³ĢŹ½£ŗ ”£
£Ø3£©ģŃÉÕ10 tÉĻŹö»ĘĢśæó£¬ĄķĀŪÉĻ²śÉśSO2µÄĢå»ż£Ø±ź×¼×“æö£©ĪŖ £¬ÖʵĆ98£„µÄĮņĖįÖŹĮæĪŖ t £¬SO2Č«²æ×Ŗ»ÆĪŖH2SO4Ź±·Å³öµÄČČĮæŹĒ kJ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012ÄźČĖ½Ģ°ęøßÖŠ»ÆѧєŠŽ2 1.1»Æ¹¤Éś²ś¹ż³ĢÖŠµÄ»ł±¾ĪŹĢāĮ·Ļ°¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ¼ĘĖćĢā
»ĘĢśæóÖ÷ŅŖ³É·ÖŹĒFeS2 ”£Ä³ĮņĖį³§ŌŚ½ųŠŠ»ĘĢśæó³É·Ö²ā¶ØŹ±£¬Č”0.1000 gѳʷŌŚæÕĘųÖŠ³ä·Ö×ĘÉÕ£¬½«Éś³ÉµÄSO2ĘųĢåÓė×ćĮæFe2(SO4)3ČÜŅŗĶźČ«·“Ó¦ŗó£¬ÓĆÅضČĪŖ0.02000 mol£ÆLµÄK2Cr2O7±ź×¼ČÜŅŗµĪ¶ØÖĮÖÕµć£¬ĻūŗÄK2Cr2O7ČÜŅŗ25.00 mL ”£
ŅŃÖŖ£ŗSO2 + 2Fe3£« + 2H2O == SO2£ + 2Fe2£« + 4H£«
Cr2O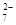 + 6
Fe2£« + 14 H£«
== 2 Cr3£« + 6 Fe3£« + 7 H2O
+ 6
Fe2£« + 14 H£«
== 2 Cr3£« + 6 Fe3£« + 7 H2O
¢Å.ѳʷ֊FeS2µÄÖŹĮæ·ÖŹżŹĒ£Ø¼ŁÉčŌÓÖŹ²»²Ī¼Ó·“Ó¦£©________________”£
¢Ę.Čō×ĘÉÕ6 g FeS2²śÉśµÄSO2Č«²æ×Ŗ»ÆĪŖSO3ĘųĢåŹ±·Å³ö9.83 kJČČĮ棬²śÉśµÄSO3ÓėĖ®Č«²æ»ÆŗĻÉś³ÉH2SO4 £¬·Å³ö13.03 kJČČĮ棬Š“³öSO3ĘųĢå×Ŗ»ÆĪŖH2SO4µÄČČ»Æѧ·½³ĢŹ½£ŗ____________________________________”£
¢Ē.ģŃÉÕ10 tÉĻŹö»ĘĢśæó£¬ĄķĀŪÉĻ²śÉśSO2µÄĢå»ż£Ø±ź×¼×“æö£©ĪŖ_______________L£¬ÖʵĆ98£„µÄĮņĖįÖŹĮæĪŖ__________ t £¬SO2Č«²æ×Ŗ»ÆĪŖH2SO4Ź±·Å³öµÄČČĮæŹĒ_______ kJ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com