
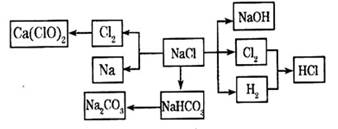
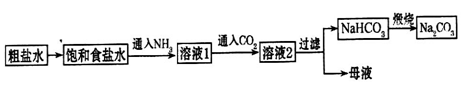
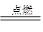 2HCl
2HCl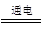 2OH����H2����Cl2��
2OH����H2����Cl2��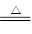 Na2CO3��H2O��CO2��
Na2CO3��H2O��CO2��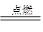 2HCl
2HCl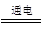 2OH����H2����Cl2��
2OH����H2����Cl2��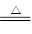 Na2CO3��H2O��CO2��
Na2CO3��H2O��CO2��

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��25��ʱ��NaHCO3��ˮ�е��ܽ�ȱ�Na2CO3�Ĵ� |
| B�������£�����������������Ӧ |
| C����������FeCl2��Һ�����պ�õ�FeO |
| D��Ư��ʹijЩȾ����ɫ�������ڻ�ѧ�仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����Ʒ�������ϡ������ | B�����Ʒ�������ˮ�� |
| C�����Ʒ�������ͭ��Һ�� | D���������������ò���ЩС�ף��ٷ���ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
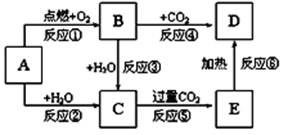
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NaCl | B��NaOH | C��BaCl2 | D��NaHCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��0.8molNa2CO3��0.6molNaOH | B��0.5molNa2CO3��1molNaOH |
| C��0.8molNa2CO3��1molNaOH | D��1molNa2CO3��0.6molNaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na2O | B��Na2O2 | C��NaOH | D��Na2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
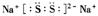 ��S32-�ĵ���ʽΪ ����֪Na2S3+2HCl = 2NaCl+H2S��+2S������д��Na2S5����ᷴӦ�����ӷ���ʽ�� ����ҵ�ϳ��õ������NaCl��Na����ʵ�ϵ���������ڵ��ƵĻ�����Ҳ���Ʊ�Na����NaOH��Na2CO3����д���������NaOH�ķ�Ӧ����ʽ�� �����������Na2CO3ʱ��CO2����������������缫��ӦʽΪ ��
��S32-�ĵ���ʽΪ ����֪Na2S3+2HCl = 2NaCl+H2S��+2S������д��Na2S5����ᷴӦ�����ӷ���ʽ�� ����ҵ�ϳ��õ������NaCl��Na����ʵ�ϵ���������ڵ��ƵĻ�����Ҳ���Ʊ�Na����NaOH��Na2CO3����д���������NaOH�ķ�Ӧ����ʽ�� �����������Na2CO3ʱ��CO2����������������缫��ӦʽΪ ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com