
����ͼʾ��գ�

H�ǻ�״������C4H6O2��F��̼ԭ����һ��ֱ���ϡ�
��1��������A���еĹ�������__________��
��2��B��������������Br2��Ӧ�õ�E��E���������������ƴ���Һ������ת��ΪF����Eת��ΪFʱ�������ַ�Ӧ���䷴Ӧ���ͷֱ���__________��
��3��D�Ľṹ��ʽ��_____________________________________________��
��4��1 mol A��2 mol H2��Ӧ����1 mol E���䷴Ӧ����ʽ��__________________________��
��5����A������ͬ�����ŵ�A��ͬ���칹��Ľṹ��ʽ��____________________________��
��1��ȩ�����Ȼ���̼̼˫�� ��2����ȥ��Ӧ���кͷ�Ӧ
��3��NaOOC��CH=CH��COONa
��4��OHC��CH==CH��COOH+2H2![]() HO��CH2CH2CH2��COOH
HO��CH2CH2CH2��COOH
![]()
A����������Һ��Ӧ��˵��A���С�CHO��������NaHCO3��Ӧ����A�л��С�COOH����A��H2��Ӧ����Gʱ����CHOת��Ϊ��CH2OH��������С�COOH�����С�OH�������������£�����������Ӧ�����ɻ�������C4H6O2������ΪF��̼ԭ����һ��ֱ���ϣ�˵��F�д���C��C������4��Cԭ����֧���ṹ������HΪ![]() ��GΪCH2OHCH2CH2CH2COOH�����ݣ�4����1 mol A��2 mol H2��Ӧ�����С�CHO��1 mol H2����˵��A�л���һ��C==C�����AΪOHC��CH==CHCOOH��BΪHOOC��CH==CH��COOH��DΪNaOOC��CH==CH��COONa��CΪOHC��CH==CH��COONa�ڴ�����Һ�У�������ȥ���кͷ�Ӧ�����ɵ�FΪNaOO��C��C��COONa������Ϊ��CHO�롪COOH������̼�����ˣ�����CHO����COOH������Ϊȡ�����ţ����Ƶ�C==C����һ��̼ԭ���ϣ�����A��ͬ���칹�壨������ͬ�����ţ�Ϊ
��GΪCH2OHCH2CH2CH2COOH�����ݣ�4����1 mol A��2 mol H2��Ӧ�����С�CHO��1 mol H2����˵��A�л���һ��C==C�����AΪOHC��CH==CHCOOH��BΪHOOC��CH==CH��COOH��DΪNaOOC��CH==CH��COONa��CΪOHC��CH==CH��COONa�ڴ�����Һ�У�������ȥ���кͷ�Ӧ�����ɵ�FΪNaOO��C��C��COONa������Ϊ��CHO�롪COOH������̼�����ˣ�����CHO����COOH������Ϊȡ�����ţ����Ƶ�C==C����һ��̼ԭ���ϣ�����A��ͬ���칹�壨������ͬ�����ţ�Ϊ![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
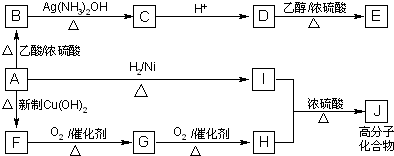
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

H�ǻ�״������C4H6O2��F��̼ԭ����һ��ֱ���ϡ�
(1)������A���еĹ�������__________��
(2)B��������������Br2��Ӧ�õ�E��E���������������ƴ���Һ������ת��ΪF����Eת��ΪFʱ�������ַ�Ӧ���䷴Ӧ���ͷֱ���__________��
(3)D�Ľṹ��ʽ��_____________________________________________��
(4)1 mol A��2 mol H2��Ӧ����1 mol E���䷴Ӧ����ʽ��__________________________��
(5)��A������ͬ�����ŵ�A��ͬ���칹��Ľṹ��ʽ��____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼʾ���

H�ǻ�״������C4H6O2 F��̼ԭ����һ��ֱ����
��1��������A���еĹ������� ��
��2��B��������������Br2��Ӧ�õ�E��E���������������ƴ���Һ������ת��ΪF����Eת��ΪFʱ�������ַ�Ӧ���䷴Ӧ���ͷֱ��� ��
��3��D�Ľṹ��ʽ�� ��
��4��1mol A��2mol H2��Ӧ����1mol G���䷴Ӧ����ʽ�� ��
��5����A������ͬ�����ŵ�A��ͬ���칹��Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼʾ���

H�ǻ�״������C4H6O2 F��̼ԭ����һ��ֱ����
��1��������A���еĹ������� ��
��2��B��������������Br2��Ӧ�õ�E��E���������������ƴ���Һ������ת��ΪF����Eת��ΪFʱ�������ַ�Ӧ���䷴Ӧ���ͷֱ��� ��
��3��D�Ľṹ��ʽ�� ��
��4��1mol A��2mol H2��Ӧ����1mol G���䷴Ӧ����ʽ�� ��
��5����A������ͬ�����ŵ�A��ͬ���칹��Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com