
Ēā»ÆøĘ¹ĢĢåŹĒµĒɽŌĖ¶ÆŌ±³£ÓƵÄÄÜŌ“Ģį¹©¼Į”£Ä³ŠĖȤŠ”×éÄāŃ”ÓĆČēĻĀ×°ÖĆÖʱøĒā»ÆøĘ”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¼ģ²é×°ÖĆEĘųĆÜŠŌµÄ²Ł×÷·½·ØŹĒ ”£
£Ø2£©ĄūÓĆÉĻŹö×°ÖĆÖĘČ”Ēā»ÆøĘŹ±°“ĘųĮ÷·½ĻņĮ¬½ÓĖ³ŠņĪŖi”ś___”ś___”ś___”ś ”ś ”ś ”śa£ØĢīŅĒĘ÷½ÓæŚµÄ×ÖÄø±ąŗÅ£©”£
£Ø3£©øł¾ŻĶźÕūµÄŹµŃé×°ÖĆ½ųŠŠŹµŃ飬ŹµŃé²½ÖčČēĻĀ£ŗ¼ģ²é×°ÖĆĘųĆÜŠŌŗó£¬×°ČėŅ©Ę·£»“ņæŖ·ÖŅŗĀ©¶·»īČū£»__________________£ØĒė°“ÕżČ·µÄĖ³ŠņĢīČėĻĀĮŠ²½ÖčµÄ±źŗÅ£©”£
| A£®¼ÓČČ·“Ó¦Ņ»¶ĪŹ±¼ä | B£®ŹÕ¼ÆĘųĢå²¢¼ģŃéĘä“æ¶Č |
| C£®¹Ų±Õ·ÖŅŗĀ©¶·»īČū | D£®Ķ£Ö¹¼ÓČČ£¬³ä·ÖĄäČ“ |
£Ø10·Ö£©£ØĆææÕ2·Ö£©
£Ø1£©·½·ØŅ»£ŗ¹Ų±ÕiæŚ£¬“ņæŖ·ÖŅŗĀ©¶·»īČū£¬“Ó·ÖŅŗĀ©¶·ÖŠ¼ÓČėÕōĮóĖ®£¬ČōÕōĮóĖ®²»ÄÜĮ÷ĻĀ£¬Ōņ×°ÖĆĘųĆÜŠŌĮ¼ŗĆ”£
·½·Ø¶ž£ŗÓƵ¼¹ÜĮ¬½ÓiæŚ£¬²¢½«µ¼¹ÜĮķŅ»¶Ė·ÅČėĖ®ÖŠ£¬Ī¢ČČŹŌ¹Ü£¬Čōµ¼¹ÜæŚÓŠĘųÅŻĆ°³ö£¬Ōņ×°ÖĆĘųĆÜŠŌĮ¼ŗĆ”£
ĄäČ“ŗóµ¼¹ÜÖŠŠĪ³ÉŅ»¶ĪĖ®Öł£¬Ōņ×°ÖĆĘųĆÜŠŌĮ¼ŗĆ”££Ø2·Ö£¬ĘäĖü·½·ØÕżČ·Ņ²æÉµĆ·Ö£©
£Ø2£©i”śe£¬f”śd£¬c”śj£¬k£Ø»ņk£¬j£©”śa £Ø2·Ö£¬Ö»ŅŖÓŠ1“¦“ķ0·Ö£©
£Ø3£©BADC£Ø2·Ö£©
£Ø4£©CaH2£«2H2O=Ca(OH)2£«2H2”ü £Ø2·Ö£© Ēā»ÆøĘŹĒ¹ĢĢ壬ŠÆ“ų·½±ć£Ø2·Ö£©
½āĪö


 æĪĢĆČ«½ā×Ö“Ź¾ä¶ĪĘŖÕĀĻµĮŠ“š°ø
æĪĢĆČ«½ā×Ö“Ź¾ä¶ĪĘŖÕĀĻµĮŠ“š°ø ²½²½øßæŚĖćĢāæØĻµĮŠ“š°ø
²½²½øßæŚĖćĢāæØĻµĮŠ“š°ø µć¾¦ŠĀ½Ģ²ÄČ«Äܽā¶ĮĻµĮŠ“š°ø
µć¾¦ŠĀ½Ģ²ÄČ«Äܽā¶ĮĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
Ä³ŃŠ¾æŠŌѧĻ°Š”×éĪŖ²ā¶ØNH3·Ö×ÓÖŠµŖ”¢ĒāŌ×ÓøöŹż±Č£¬Éč¼ĘČēĻĀŹµŃéĮ÷³Ģ£ŗ
ŹµŃ鏱£¬ĻČÓĆÖʵƵݱĘųÅž”Ļ“ĘųĘæĒ°ĖłÓŠ×°ÖĆÖŠµÄæÕĘų£¬ŌŁĮ¬½ÓĻ“ĘųĘæŗĶĘųĢåŹÕ¼Æ×°ÖĆ£¬Į¢¼“¼ÓČČŃõ»ÆĶ”£
ĻĀĶ¼A”¢B”¢CĪŖøĆŠ”×éÖĘČ”°±ĘųŹ±æÉÄÜÓƵ½µÄ×°ÖĆ£¬DĪŖŹ¢ÓŠÅØĮņĖįµÄĻ“ĘųĘ攣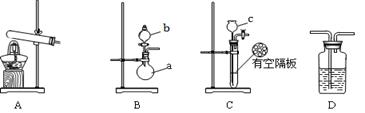
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öŅĒĘ÷µÄĆū³Ę£ŗa £¬b ”£
£Ø2£©Ó²ÖŹ²£Į§¹ÜÖŠ·¢ÉśµÄ·“Ó¦·½³ĢŹ½ŹĒ ””£¬·“Ó¦¹ż³ĢÖŠÓ²ÖŹ²£Į§¹ÜµÄĻÖĻóŹĒ ”£
£Ø3£©ĒėÅŠ¶ĻÖĘČ”°±ĘųæÉÄÜÓƵ½µÄ×°ÖĆ£¬ŌŚĻĀ±ķÖŠÄćČĻĪŖæÉŠŠµÄ×°ÖĆÖŠĢīŠ“¶ŌÓ¦µÄŹµŃéŅ©Ę·£ØŠ“³ö»ÆѧŹ½£©”£
| ×°ÖĆ | ŹµŃéŅ©Ę· |
| A | |
| B | b£ŗ a£ŗ |
| C | c£ŗ øō°å£ŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ĻÖÄāŌŚŹµŃéŹŅĄļĄūÓĆæÕĘųŗĶĆ¾·ŪĪŖŌĮĻÖĘȔɣĮæµŖ»ÆĆ¾(Mg3N2)”£ŅŃÖŖŹµŃéÖŠæÉÄÜ»į·¢ÉśĻĀĮŠ·“Ó¦£ŗ
¢Ł2Mg+O2 2MgO£»¢Ś3Mg+N2
2MgO£»¢Ś3Mg+N2 Mg3N2£»¢Ū2Mg+CO2
Mg3N2£»¢Ū2Mg+CO2 2MgO+C£»
2MgO+C£»
¢ÜMg+H2O MgO+H2”ü£» ¢ŻMg3N2 +6H2O
MgO+H2”ü£» ¢ŻMg3N2 +6H2O 3Mg(OH)2+2NH3”ü
3Mg(OH)2+2NH3ӟ
æɹ©Ń”ŌńµÄ×°ÖĆŗĶŅ©Ę·ČēĻĀĶ¼ĖłŹ¾(Ć¾·Ū”¢»¹ŌĢś·Ū¾łŅŃøÉŌļ£¬×°ÖĆÄŚĖł·¢ÉśµÄ·“Ó¦ŹĒĶźČ«µÄ£¬ÕūĢ××°ÖƵÄÄ©¶ĖÓėøÉŌļ¹ÜĻąĮ¬)”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŌŚÉč¼ĘŹµŃé·½°øŹ±£¬³ż×°ÖĆA”¢D”¢EĶā£¬»¹Ó¦Ń”ŌńµÄ×°ÖĆÓŠ £ØĢī×ÖÄø“śŗÅ£©£¬Ń”Ōń×°ÖĆDÄæµÄĪŖ_____________________________ £»
£Ø2£©ĶØĘųŗó£¬Ó¦ĻȵćČ¼ “¦µÄ¾Ę¾«µĘ£»Čē¹ūĶ¬Ź±µćČ¼A”¢F×°ÖĆµÄ¾Ę¾«µĘ£¬½«»įŹ¹ŹµŃé½į¹ū £ØĢī”°Ę«øß”±»ņ”°Ę«µĶ”±£©ŌŅņ
£Ø3£©ĒėÉč¼ĘŅ»øöŹµŃ飬ŃéÖ¤²śĪļŹĒMg3N2£¬Š“³ö²Ł×÷²½Öč”¢ĻÖĻóŗĶ½įĀŪ£ŗ
_____________________________________________________________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
SO2ŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬Ņ²ŹĒŅżĘšĖįÓźĪŪČ¾µÄÖŲŅŖĄ“Ō“”£
(1)ijŠĖȤŠ”×é²ÉÓĆČēĶ¼ĖłŹ¾×°ÖĆÖĘČ”SO2
¢ŁĻĀĮŠŹµŃé·½°øŹŹÓĆČēĶ¼ĖłŹ¾×°ÖĆÖĘČ”ĖłŠčSO2µÄŹŌ¼ĮŹĒ_______(ĢīŠņŗÅ£©”£
| A£®Na2SO3ČÜŅŗÓėĻ”ĻõĖį |
| B£®Na2SO3¹ĢĢåÓėÅØĮņĖį |
| C£®¹ĢĢåĮņŗĶŃõĘų |
| D£®ĶÓėÅØĮņĖį |
 Éś²śĮ÷³Ģ£¬ĘäĮ÷³ĢŹ¾ŅāĶ¼ČēĻĀ£ŗ
Éś²śĮ÷³Ģ£¬ĘäĮ÷³ĢŹ¾ŅāĶ¼ČēĻĀ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
(14·Ö)ij»Æѧ»ī¶ÆŠ”×éÉč¼ĘČēĻĀĶ¼ĖłŹ¾(²æ·Ö¼Š³Ö×°ÖĆŅŃĀŌČ„)ŹµŃé×°ÖĆ£¬ŅŌĢ½¾æ³±ŹŖµÄCl2ÓėNa2CO3·“Ó¦µĆµ½µÄ¹ĢĢåĪļÖŹ”£
£Ø1£©×°ÖĆAÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ__________________________________________”£
£Ø2£©×°ÖĆBÖŠŹŌ¼ĮYÓ¦ĪŖ__________________________________”£
£Ø3£©øĆŹµŃé×°ÖĆÖŠĆ÷ĻŌ“ęŌŚ²»×ćÖ®“¦£¬øĽųµÄ“ėŹ©ĪŖ_____________________________”£
£Ø4£©ŅŃÖŖŌŚ×°ÖĆCÖŠĶØČėŅ»¶ØĮæµÄĀČĘųŗ󣬲āµĆDÖŠÖ»ÓŠŅ»ÖÖ³£ĪĀĻĀĪŖ»ĘŗģÉ«µÄĘųĢå(ŗ¬ĀČŃõ»ÆĪļ)”£CÖŠŗ¬ĀČŌŖĖŲµÄŃĪÖ»ÓŠŅ»ÖÖ£¬ĒŅŗ¬ÓŠNaHCO3”£ĻÖ¶ŌCÖŠµÄ³É·Ö½ųŠŠ²ĀĻėŗĶĢ½¾æ”£
¢ŁĢį³öŗĻĄķ¼ŁÉč”£
¼ŁÉčŅ»£ŗ“ęŌŚĮ½Öֳɷ֣¬ĪŖNaHCO3ŗĶ____________£»
¼ŁÉ趞£ŗ“ęŌŚČżÖֳɷ֣¬ĪŖNaHCO3ŗĶ_____________”¢______________”£
¢ŚÉč¼Ę·½°ø²¢ŹµŃ锣ĒėŌŚ±ķøńÖŠŠ“³öŹµŃé²½ÖčŅŌ¼°Ō¤ĘŚĻÖĻóŗĶ½įĀŪ”£
ĻŽŃ”ŹŌ¼ĮŗĶŅĒĘ÷£ŗÕōĮóĖ®”¢Ļ”ĻõĖį”¢BaCl2ČÜŅŗ”¢³ĪĒåŹÆ»ŅĖ®”¢AgNO3ČÜŅŗ”¢ŹŌ¹Ü”¢Š”ÉÕ±£®
½įĀŪ£ŗÓɲ½Öč3µÄ½įĀŪ½įŗĻ²½Öč2ÖŠµÄa£¬Ōņ¼ŁÉčŅ»³ÉĮ¢£»Óɲ½Öč3µÄ½įĀŪ½įŗĻ²½Öč2ÖŠµÄb£¬Ōņ¼ŁÉ趞³ÉĮ¢”£
£Ø5£©ŅŃÖŖCÖŠÓŠ0.1 mol Cl2²Ī¼Ó·“Ó¦”£Čō¼ŁÉčŅ»³ÉĮ¢£¬æÉĶĘÖŖCÖŠ·“Ӧɜ³ÉµÄŗ¬ĀČŃõ»ÆĪļĪŖ_________(Š“»ÆѧŹ½)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
Ņ½ÓĆĀČ»ÆøĘæÉÓĆÓŚ²¹øĘ”¢æ¹¹żĆōŗĶĻūŃ×µČ£¬ŅŌ¹¤ŅµĢ¼ĖįøĘ£Øŗ¬ÉŁĮæNa+”¢Al3+”¢Fe3+µČŌÓÖŹ£©Éś²śŅ½ÓƶžĖ®ŗĻĀČ»ÆøĘ¹¤ŅÕĮ÷³ĢĪŖ£ŗ 
ŅŃÖŖ£ŗ²éŌÄ׏ĮĻµĆÖŖĒāŃõ»ÆĪļ³ĮµķŹ±µÄpHĪŖ£ŗ
| ĒāŃõ»ÆĪļ | Fe(OH)3 | Al(OH)3 | |
| æŖŹ¼³ĮµķŹ±µÄpH | 2.3 | 4.0 | æŖŹ¼Čܽā£ŗ7.8 |
| ĶźČ«³ĮµķŹ±µÄpH | 3.7 | 5.2 | ĶźČ«Čܽā£ŗ10.8 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀ±ķÖŠµÄŹµŃé²Ł×÷ÄÜ“ļµ½ŹµŃéÄæµÄ»ņÄÜµĆ³öĻąÓ¦½įĀŪµÄŹĒ
| Ń”Ļī | ŹµŃéÄŚČŻ | ŹµŃéÄæµÄ»ņŹµŃé½įĀŪ |
| A | ĻņŹ¢ÓŠ2 mL 0.1 mol/L AgNO3ČÜŅŗµÄŹŌ¹ÜÖŠµĪ¼Ó5µĪ0.1 mol/L NaClČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É£¬ŌŁĻņĘäÖŠµĪ¼Ó5µĪ0.1 mol/L KIČÜŅŗ | ĖµĆ÷Ņ»ÖÖ³ĮµķÄÜ×Ŗ»ÆĪŖČܽā¶ČøüŠ”µÄ³Įµķ |
| B | Ļņ1 mL 20% µÄÕįĢĒČÜŅŗÖŠ¼ÓČė3”«5µĪĻ”ĮņĖį£¬Ė®Ō”¼ÓČČ5 min£¬ĄäČ“ŗóŌŁ¼ÓČėŠĀÖĘCu(OH)2Šü×ĒŅŗ£¬¼ÓČČ | Ö¤Ć÷ÕįĢĒÄÜ·¢ÉśĖ®½ā·“Ó¦ |
| C | Ė®Ō”¼ÓČČÅØĻõĖį”¢ÅØĮņĖįŗĶ±½µÄ»ģŗĻĪļŗó£¬Ö±½ÓÕōĮó·ÖŅŗŗóµĆµ½µÄ“Ö²śĘ· | Öʱø“æĻõ»ł±½ |
| D | ŹŅĪĀĻĀ,·Ö±šĻņ2Ö§ŹŌ¹ÜÖŠ¼ÓČėĻąĶ¬Ģå»ż”¢ĻąĶ¬ÅØ¶ČµÄNa2S2O3ČÜŅŗ,ŌŁ·Ö±š¼ÓČėµČĢå»ż²»Ķ¬ÅØ¶ČµÄĻ”ĮņĖį | ŃŠ¾æÅØ¶Č¶Ō·“Ó¦ĖŁĀŹµÄÓ°Ļģ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ČēĶ¼ĖłŹ¾ŹĒŅ»Ģ׏µŃéŹŅÖĘĘų×°ÖĆ£¬ÓĆÓŚ·¢Éś”¢øÉŌļŗĶŹÕ¼ÆĘųĢ唣ĻĀĮŠø÷×éĪļÖŹÖŠÄÜĄūÓĆÕāĢ××°ÖĆ½ųŠŠŹµŃéµÄŹĒ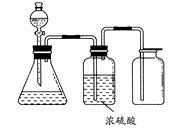
| A£®ĶŠ¼ŗĶĻ”ĻõĖį |
| B£®¶žŃõ»ÆĆĢŗĶÅØŃĪĖį |
| C£®ÓĆÅØ°±Ė®ŗĶÉśŹÆ»Ņ·“Ó¦ |
| D£®Ģ¼ĖįøĘŗĶĻ”ŃĪĖį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀŹöŹµŃéÄÜ“ļµ½Ō¤ĘŚŹµŃéÄæµÄµÄŹĒ£Ø £©
| ŠņŗÅ | ŹµŃéÄŚČŻ | ŹµŃéÄæµÄ |
| A | ŹŅĪĀĻĀ,ÓĆpHŹŌÖ½²ā¶ØÅضČĪŖ0.1 mol”¤L-1NaClOČÜŅŗŗĶ0.1 mol”¤L-1 CH3COONaČÜŅŗµÄpH | ±Č½ĻHClOŗĶCH3COOHµÄĖįŠŌĒæČõ |
| B | ĻņŹ¢ÓŠ1 mLĻõĖįŅųČÜŅŗµÄŹŌ¹ÜÖŠµĪ¼ÓNaClČÜŅŗ,ÖĮ²»ŌŁÓŠ³ĮµķÉś³É,ŌŁĻņĘäÖŠµĪ¼ÓNa2SČÜŅŗ | ĖµĆ÷Ņ»ÖÖ³ĮµķÄÜ×Ŗ»ÆĪŖĮķŅ»ÖÖČܽā¶ČøüŠ”µÄ³Įµķ |
| C | ĻņNaAlO2ČÜŅŗÖŠµĪ¼Ó±„ŗĶNaHCO3ČÜŅŗ,ÓŠ°×É«³Įµķ²śÉś | ŃéÖ¤Į½Õ߶¼·¢ÉśĮĖĖ®½ā·“Ó¦,ĒŅĻą»„“Ł½ų |
| D | ŹŅĪĀĻĀ,·Ö±šĻņ2Ö§ŹŌ¹ÜÖŠ¼ÓČėĻąĶ¬Ģå»ż”¢ĻąĶ¬ÅØ¶ČµÄNa2S2O3ČÜŅŗ,ŌŁ·Ö±š¼ÓČė²»Ķ¬ÅØ¶ČµÄĻ”ĮņĖį | ŃŠ¾æÅØ¶Č¶Ō·“Ó¦ĖŁĀŹµÄÓ°Ļģ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com