
���� ��1����Һ��������Ũ��Խ����Һ��pHԽС����Һ������������Ũ��Խ����Һ��pHԽ�ݴ˽����жϣ�
��2��������������ƶ���ǿ����ʣ�����Һ�������Ӻ�����������Ũ����ȣ����Һ��pH=7��������Һ�����ȣ�
��3��pH=13������������Һ��Ũ��Ϊ0.1mol/L������Һ��������ǡ�÷�Ӧ���ɴ����ƣ���������Ӳ���ˮ�⣬��Һ�ʼ��ԣ���ϵ���غ��жϸ�����Ũ�ȴ�С��
��� �⣺��1����0.1mol/L CH3COOH��Һ��������Ũ��С��0.1mol/L����Һ��pH��1����pH=13NaOH��Һ����0.05mol/L H2SO4��Һ��������Ũ��Ϊ0.1mol/L����ҺpH=1����0.1mol/L Na2CO3��Һ�������ԣ���������Һ��pH�ɴ�СΪ���ڢܢ٢ۣ�
�ʴ�Ϊ���ڢܢ٢ۣ�
��2����pH=13NaOH��Һ������������Ũ��Ϊ0.1mol/L����0.05mol/L H2SO4��Һ��������Ũ��Ϊ0.1mol/L�����Һ��pH=7������������������ƶ���ǿ����ʣ�������Һ�����ȣ�����a��b=1��1��
�ʴ�Ϊ��1��1��
��3�������¶�����Һ������Һ�ڵ������Ϻ�����Ϊ�����ƣ���������Ӳ���ˮ�⣬��Һ�ʼ��ԣ���c��OH-����c��H+�������ݵ���غ��֪c��Na+����c��CH3COO-��������Һ������Ũ�ȴ�СΪ��c��Na+����c��CH3COO-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��CH3COO-����c��OH-����c��H+����
���� ���⿼��������ϵĶ����жϼ���ҺpH�ļ��㣬��Ŀ�Ѷ��еȣ��漰������ʵĵ���ƽ�⡢����ϵĶ����жϡ���ҺpH�ļ����֪ʶ������֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ���ѧ���ķ������������������Ӧ��������ע����������Ũ�ȴ�С�Ƚϵij��÷�������ȷ����ϵĶ����жϷ�������ҺpH�ļ��㷽����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 7�� | B�� | 8�� | C�� | 9�� | D�� | 10�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | �׳��ǵ��أ��ҳ���ԭ��أ�A�缫��ӦʽΪ��C2H5OH+3H2O-12e-�T2CO2+12H+ | |
| B�� | ��Ӧһ��ʱ���������Һ��pH��δ�仯 | |
| C�� | �����ҳ��м���K2SO4��Һ����Ĥֻ����K+ͨ��������·��ת��0.01mol e-ʱ�����Ĥ�����Һ�����ռ�������Լ0.02mol | |
| D�� | �����ҳ��м���NaI��Һ�������ҳط�Ӧ�����У����Թ۲쵽C�缫��Χ����Һ�����ػ�ɫ����Ӧ��Ϻ��ò�����������Һ�����²���Һ�����Ϻ�ɫ���ϲ�ӽ���ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
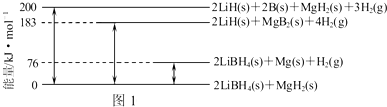
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol | B�� | 2mol | C�� | 3mol | D�� | 4mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
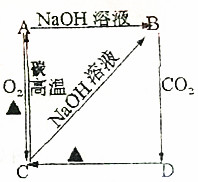 ������ͼ��ʾ�仯��ϵ����ش��������⣮
������ͼ��ʾ�仯��ϵ����ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1��3 | B�� | 1��2 | C�� | 2��3 | D�� | 3��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
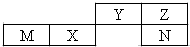 ���ֶ�����Ԫ����Ԫ�����ڱ��е�λ����ͼ������ֻ��һ��Ϊ����Ԫ�أ�����˵����ȷ���ǣ�������
���ֶ�����Ԫ����Ԫ�����ڱ��е�λ����ͼ������ֻ��һ��Ϊ����Ԫ�أ�����˵����ȷ���ǣ�������| A�� | �����Ӱ뾶��С��M��N��Z | |
| B�� | �����̬�⻯��ķе�ߵͣ�N��Z��Y | |
| C�� | M��������������N������������Ӧˮ���ﷴӦ | |
| D�� | N���ʿ���Z������⻯�ﷴӦ�û���Z���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
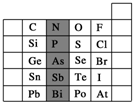 Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʣ���ͼ��Ԫ�����ڱ���һ���֣�
Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʣ���ͼ��Ԫ�����ڱ���һ���֣��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com