

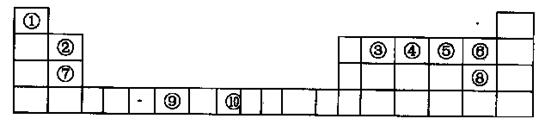
 ���������к��еĻ�ѧ�������� ������ĸ��ţ���
���������к��еĻ�ѧ�������� ������ĸ��ţ��� ��E+δ������������ɫ�����һ��������E+�ĸ���Ϊ ����
��E+δ������������ɫ�����һ��������E+�ĸ���Ϊ ����
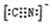 4
4  X4��F3+Ϊ
X4��F3+Ϊ X4��AB-Ϊ
X4��AB-Ϊ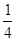 X12=3�����ݵ��������Ϊ0��ԭ����ɫ���徧����
X12=3�����ݵ��������Ϊ0��ԭ����ɫ���徧���� ��E+δ��������
��E+δ�������� ���е�E+Ϊ
���е�E+Ϊ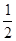 �������ɫ�����һ��������E+�ĸ���Ϊ8x1/2="4"
�������ɫ�����һ��������E+�ĸ���Ϊ8x1/2="4" 

 �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
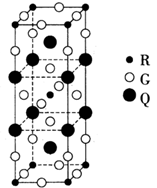
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| A��NaN3��KN3�ṹ���ƣ�ǰ�߾����ܽ�С |
B������صľ����ṹ��ͼ��ʾ�� ��ÿ�������з�̯2����ԭ�� ��ÿ�������з�̯2����ԭ�� |
| C�����ĵ�һ�����ܴ����� |
| D�����������º��ȶ�������Ϊ���ĵ縺��С |
 Rx[CrCln(H2O)6��n]+xH+������0.0015 mol[CrCln(H2O)6��n]x������Һ����R-H��ȫ�������к����ɵ�H+��Ũ��Ϊ0.1200 mol��L-1 NaOH��Һ25.00 mL����������ӵĻ�ѧʽΪ_______��
Rx[CrCln(H2O)6��n]+xH+������0.0015 mol[CrCln(H2O)6��n]x������Һ����R-H��ȫ�������к����ɵ�H+��Ũ��Ϊ0.1200 mol��L-1 NaOH��Һ25.00 mL����������ӵĻ�ѧʽΪ_______���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
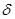 ����
����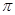 ���ĸ�����Ϊ ��
���ĸ�����Ϊ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com