
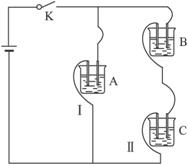
��֪A��B��C�ֱ�ѡ��������Һ��0.1 mol��L-1���ᡢ0.1 mol��L-1���ᡢ0.1 mol��L-1 NaCl��Һ��0.1 mol��L-1 NaOH��Һ��0.1 mol��L-1��ˮ����25 ��ʱ��A��ҺpH��7��
����������⣺
��1��ָ��A��B��C�ǣ�������ǣ�ʲô��Һ��
A.______________��B.______________��C.______________��
��2������C��Һ�е����̪�Լ��ʺ�ɫ����C��________����A��B��C�ֱ��Ե��������������ϣ������________��ϵĻ��Һ�У�ˮ�ĵ���̶����ѡ��A��B��C�ش𣩡�
��1��0.1 mol��L-1���� 0.1 mol��L-1��ˮ �����NaCl��Һ��NaOH��Һ
��2��NaOH��Һ AB
�������ڵ�ѹ���ʱ������Ũ��Խ������Һ�ĵ�����Խǿ����·�ĵ���Խ�պ�S��I����I������ȥB��IA����IC��˵��A��B�����������Һ��C��ǿ�������Һ��IAB����IA��˵��A��B��Ϻ�����ǿ����ʡ���֪��Һ��ֻ�д�����Һ�Ͱ�ˮ�����������Һ������ΪA��Һ��pH��7����A�Ǵ�����Һ��B�ǰ�ˮ�����ڴ������ˮ�⣬����Һ��ˮ�ĵ���̶����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

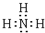
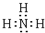
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
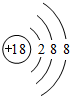
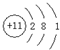
��A��B��C���ֶ�����Ԫ�أ����ǵ�ԭ��������������BԪ��ԭ��������������CԪ��ԭ��������������һ�룬AԪ��ԭ��������������BԪ��ԭ��������������һ�����ס��ҡ���������A��B��C��Ԫ����ۺ���������Σ��ס�����ҺpH>7������ҺpH<7����Ϊ���壬��Ϊ����ɫ���塣�ס��ҡ����������졢����������֮������Ӧ��ϵ����ͼ�����ֲ�������ȥ����
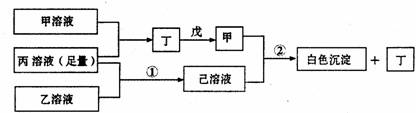
��1��A��Ԫ������Ϊ ��CԪ�صļ����ӽṹʾ��ͼΪ ��
��2��д����Ӧ�ٵ����ӷ���ʽ ��
��3������Һ���������ӵ�Ũ���ɴ�С��˳��Ϊ ��
��4��������ͭ�缫�������Һ�н��е�⣬��ʼʱ���������ӷ���ʽΪ ��
��5��д����Ӧ�ڵĻ�ѧ����ʽ .
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A��B��C���������κ�һ������D��A��B�еĽ���Ԫ��Ϊͬ�壬A��D��ˮ��Һ��Ͽ����ɻ�ɫ������B��D��ˮ��Һ��Ͽ����ɰ�ɫ������C��D��ˮ��Һ��Ͽ����ɺ�ɫ��������C����Һ�м���ǿ������������ɫ��״�������ó������ڹ�����ǿ���С��ò���������ͭ�����������D����Һ����������������ɫ������
��д��A��B��C��D�ķ���ʽ��(2��)
��д��A��B��C��D����Һ��Ӧ�����ӷ���ʽ��(3��)
��д��C��ǿ����Һ��Ӧ�����ӷ���ʽ��(2��)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com