
ijæĪĶāŃŠ¾æŠ”×飬ÓĆŗ¬ÓŠ½Ļ¶ąŌÓÖŹµÄĶ·Ū£¬Ķعż²»Ķ¬µÄ»Æѧ·“Ó¦ÖĘČ”µØ·Æ”£ĘäÉč¼ĘµÄŹµŃé¹ż³ĢĪŖ£ŗ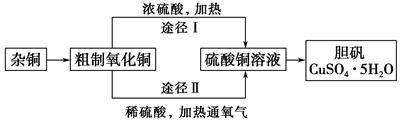
(1)ŌÓĶÖŠŗ¬ÓŠ“óĮæµÄÓŠ»śĪļ£¬æɲÉÓĆ×ĘÉյķ½·Ø³żČ„ÓŠ»śĪļ£¬×ĘÉÕŹ±½«“ÉŪįŪöÖĆÓŚ ÉĻ(ÓĆŅŌĻĀĖłøųŅĒĘ÷µÄ±ąŗÅĢīČė£¬ĻĀĶ¬)£¬Č”ÓĆŪįŪöÓ¦Ź¹ÓĆ £¬×ĘÉÕŗóµÄŪįŪöÓ¦·ÅŌŚ ÉĻ£¬²»ÄÜÖ±½Ó·ÅŌŚ×ĄĆęÉĻ”£
ŹµŃéĖłÓĆŅĒĘ÷£ŗ
aÕō·¢Ćó£»bŹÆĆŽĶų£»cĹȿ½Ē£»d±ķĆęĆó£»eŪįŪöĒÆ£»fŹŌ¹Ü¼Š
(2)ŌÓĶ¾×ĘÉÕŗóµĆµ½µÄ²śĪļŹĒŃõ»ÆĶ¼°ÉŁĮæĶµÄ»ģŗĻĪļ£¬×ĘÉÕŗóŗ¬ÓŠÉŁĮæĶµÄæÉÄÜŌŅņŹĒ ”£
a£®×ĘÉÕ¹ż³ĢÖŠ²æ·ÖŃõ»ÆĶ±»»¹Ō
b£®×ĘÉÕ²»³ä·ÖĶĪ“±»ĶźČ«Ńõ»Æ
c£®Ńõ»ÆĶŌŚ¼ÓČČ¹ż³ĢÖŠ·Ö½āÉś³ÉĶ
d£®øĆĢõ¼žĻĀĶĪŽ·Ø±»ŃõĘųŃõ»Æ
(3)ĶعżĶ¾¾¶¢ņŹµĻÖÓĆ“ÖÖĘŃõ»ÆĶÖĘČ”µØ·Æ£¬±ŲŠė½ųŠŠµÄŹµŃé²Ł×÷²½Öč£ŗĖįČÜ”¢¼ÓČČĶØŃõĘų”¢¹żĀĖ”¢ ”¢ĄäČ“½į¾§”¢ ”¢×ŌČ»øÉŌļ”£
(4)ÓÉ“ÖÖĘŃõ»ÆĶĶعżĮ½ÖÖĶ¾¾¶ÖĘČ”µØ·Æ£¬ÓėĶ¾¾¶¢ńĻą±Č£¬Ķ¾¾¶¢ņÓŠĆ÷ĻŌµÄĮ½øöÓŵćŹĒ ”¢ ”£
(5)ŌŚ²ā¶ØĖłµĆµØ·Æ(CuSO4”¤xH2O)ÖŠ½į¾§Ė®xÖµµÄŹµŃé¹ż³ĢÖŠ£ŗ³ĘĮæ²Ł×÷ÖĮÉŁ½ųŠŠ “Ī”£
(6)Čō²ā¶Ø½į¹ūxֵʫøߣ¬æÉÄܵÄŌŅņŹĒ (Ģī×ÖÄø±ąŗÅ)”£
a£®¼ÓČČĪĀ¶Č¹żøß
b£®µØ·Æ¾§ĢåµÄæÅĮ£½Ļ“ó
c£®¼ÓČČŗó·ÅŌŚæÕĘųÖŠĄäČ“
d£®µØ·Æ¾§Ģå²æ·Ö·ē»Æ

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©ČēĶ¼ŹĒ²æ·Ö¶ĢÖÜĘŚŌŖĖŲµÄµ„ÖŹ¼°Ęä»ÆŗĻĪļ£Ø»ņĘäČÜŅŗ£©µÄ×Ŗ»Æ¹ŲĻµ£¬ŅŃÖŖB”¢C”¢D”¢EŹĒ·Ē½šŹōµ„ÖŹ£¬ĒŅŌŚ³£ĪĀ³£Ń¹ĻĀ¶¼ŹĒĘųĢ壻»ÆŗĻĪļGµÄŃęÉ«·“Ó¦ĪŖ»ĘÉ«£¬»ÆŗĻĪļIŗĶJĶس£×“æöĻĀ³ŹĘųĢ¬£»·“Ó¦¢ŁŹĒ»Æ¹¤Éś²śÖŠµÄŅ»ÖÖÖŲŅŖµÄ¹ĢµŖ·“Ó¦”£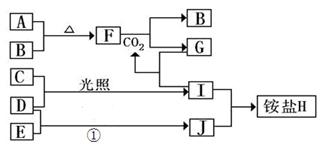
£Ø1£©µ„ÖŹEµÄ½į¹¹Ź½_____________
£Ø2£©FŗĶH2O·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_____________________________£¬øĆ·“Ó¦ÖŠ±»Ńõ»ÆÓė±»»¹ŌµÄĪļÖŹµÄĪļÖŹµÄĮæÖ®±ČŹĒ_________________
£Ø3£©ļ§ŃĪHÖŠŃōĄė×ӵĵē×ÓŹ½_______________,¼ģŃéH ÖŠŃōĄė×ӵķ½·ØŹĒ____________________
_____________________________________________________________________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¹¤ŅµÉĻÓÉĀĮĶĮæó£ØÖ÷ŅŖ³É·ÖŹĒAl2O3ŗĶFe2O3£©ŗĶ½¹ĢæÖʱøĪŽĖ®AlCl3µÄĮ÷³ĢČēĻĀ£ŗ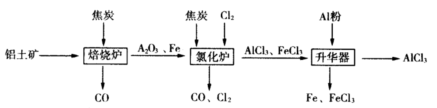
ŅŃÖŖ£ŗAlCl3£¬FeCl3£¬·Ö±šŌŚ183”ę”¢315”ęÉż»Ŗ
£Ø1£©ŌŚ±ŗÉÕĀÆÖŠ·¢Éś·“Ó¦£ŗ
¢ŁFe2O3(s)£«3C(s£© 2Fe(s)£«3CO(g£© ”÷H£½£492.7kJ/mol
2Fe(s)£«3CO(g£© ”÷H£½£492.7kJ/mol
¢Ś3CO(g)+ Fe2O3(s) 2Fe(s)£«3CO2(g£© ”÷H£½£«25.2kJ/mol
2Fe(s)£«3CO2(g£© ”÷H£½£«25.2kJ/mol
·“Ó¦2Fe2O3(s)£«3C(s) 4Fe(s)£«3CO2(g£© ”÷H£½___________kJ/mol”£
4Fe(s)£«3CO2(g£© ”÷H£½___________kJ/mol”£
£Ø2£©¢ŁAl2O3£¬Cl2ŗĶCŌŚĀČ»ÆĀÆÖŠøßĪĀĻĀ·¢Éś·“Ó¦£¬µ±Éś³É1molAlCl3Ź±×ŖŅĘ______molµē×Ó£»ĀÆĘųÖŠŗ¬ÓŠ“óĮæCOŗĶÉŁĮæCl2£¬æÉÓĆNa2SO3ČÜŅŗ³żČ„Cl2£¬ĘäĄė×Ó·½³ĢŹ½ĪŖ_______”£ŌŚĪĀ¶ČŌ¼ĪŖ700”ęĻņÉż»ŖĘ÷ÖŠ¼ÓČėĀĮ·Ū£¬·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£³ä·Ö·“Ó¦ŗóĪĀ¶Č½µÖĮ_____ŅŌĻĀ£ØĢī”°183”ę”¢315”ęÖ®Ņ»£©£¬æŖŹ¼·ÖĄėŹÕ¼ÆAlCl3”£
¢Ś½«AlCl3”¤ 6H2OČÜÓŚÅØĮņĖį½ųŠŠÕōĮó£¬Ņ²ÄܵƵ½Ņ»¶ØĮæµÄĪŽĖ®AlCl3£¬“ĖŌĄķŹĒĄūÓĆÅØĮņĖįĻĀĮŠŠŌÖŹÖŠµÄ £ØĢī×ÖÄøŠņŗÅ£©”£
¢ŁŃõ»ÆŠŌ ¢ŚĪüĖ®ŠŌ ¢ŪÄѻӷ¢ŠŌ ¢ÜĶŃĖ®ŠŌ
a£®Ö»ÓŠ¢Ł b£®Ö»ÓŠ¢Ś c£®Ö»ÓŠ¢Ś¢Ū d£®Ö»ÓŠ¢Ś¢Ū¢Ü
£Ø3£©ŗ£ŃóµĘĖžµē³ŲŹĒĄūÓĆĀĮ”¢ŹÆÄ«ĪŖµē¼«²ÄĮĻ£¬ŗ£Ė®ĪŖµē½āÖŹČÜŅŗ£¬¹¹³Éµē³ŲµÄĘäÕż¼«·“Ó¦Ź½________£¬ÓėĒ¦Šīµē³ŲĻą±Č£®ŹĶ·ÅĻąĶ¬µēĮæŹ±£¬ĖłĻūŗĽšŹōµē¼«²ÄĮĻµÄÖŹĮæ±Čm£ØAl£©: m£ØPb£©=__________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijæĪĶāŃŠ¾æŠ”×飬ÓĆŗ¬ÓŠ½Ļ¶ąŌÓÖŹµÄĶ·Ū£¬Ķعż²»Ķ¬µÄ»Æѧ·“Ó¦ÖĘČ”µØ·Æ”£ĘäÉč¼ĘµÄŹµŃé¹ż³ĢĪŖ£ŗ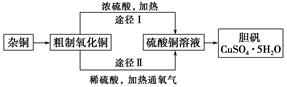
(1)ŌÓĶÖŠŗ¬ÓŠ“óĮæµÄÓŠ»śĪļ£¬æɲÉÓĆ×ĘÉյķ½·Ø³żČ„ÓŠ»śĪļ£¬×ĘÉÕŹ±½«“ÉŪįŪöÖĆÓŚ ÉĻ(ÓĆŅŌĻĀĖłøųŅĒĘ÷µÄ±ąŗÅĢīČė£¬ĻĀĶ¬)£¬Č”ÓĆŪįŪöÓ¦Ź¹ÓĆ £¬×ĘÉÕŗóµÄŪįŪöÓ¦·ÅŌŚ ÉĻ£¬²»ÄÜÖ±½Ó·ÅŌŚ×ĄĆęÉĻ”£
ŹµŃéĖłÓĆŅĒĘ÷£ŗ
a£®Õō·¢Ćó
b£®ŹÆĆŽĶų
c£®ÄąČż½Ē
d£®±ķĆęĆó
e£®ŪįŪöĒÆ
f£®ŹŌ¹Ü¼Š
(2)ŌÓĶ¾×ĘÉÕŗóµĆµ½µÄ²śĪļŹĒŃõ»ÆĶ¼°ÉŁĮæĶµÄ»ģŗĻĪļ£¬×ĘÉÕŗóŗ¬ÓŠÉŁĮæĶµÄæÉÄÜŌŅņŹĒ ”£
a£®×ĘÉÕ¹ż³ĢÖŠ²æ·ÖŃõ»ÆĶ±»»¹Ō
b£®×ĘÉÕ²»³ä·ÖĶĪ“±»ĶźČ«Ńõ»Æ
c£®Ńõ»ÆĶŌŚ¼ÓČČ¹ż³ĢÖŠ·Ö½āÉś³ÉĶ
d£®øĆĢõ¼žĻĀĶĪŽ·Ø±»ŃõĘųŃõ»Æ
(3)ĶعżĶ¾¾¶¢ņŹµĻÖÓĆ“ÖÖĘŃõ»ÆĶÖĘČ”µØ·Æ£¬±ŲŠė½ųŠŠµÄŹµŃé²Ł×÷²½Öč£ŗĖįČÜ”¢¼ÓČČĶØŃõĘų”¢¹żĀĖ”¢ ”¢ĄäČ“½į¾§”¢ ”¢×ŌČ»øÉŌļ”£
(4)ÓÉ“ÖÖĘŃõ»ÆĶĶعżĮ½ÖÖĶ¾¾¶ÖĘČ”µØ·Æ£¬ÓėĶ¾¾¶¢ńĻą±Č£¬Ķ¾¾¶¢ņÓŠĆ÷ĻŌµÄĮ½øöÓŵćŹĒ ”¢ ”£
(5)ŌŚ²ā¶ØĖłµĆµØ·Æ(CuSO4”¤xH2O)ÖŠ½į¾§Ė®xÖµµÄŹµŃé¹ż³ĢÖŠ£ŗ³ĘĮæ²Ł×÷ÖĮÉŁ½ųŠŠ “Ī”£
(6)Čō²ā¶Ø½į¹ūxֵʫøߣ¬æÉÄܵÄŌŅņŹĒ (Ģī×ÖÄø±ąŗÅ)”£
a£®¼ÓČČĪĀ¶Č¹żøß
b£®µØ·Æ¾§ĢåµÄæÅĮ£½Ļ“ó
c£®¼ÓČČŗó·ÅŌŚæÕĘųÖŠĄäČ“
d£®µØ·Æ¾§Ģå²æ·Ö·ē»Æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
“æ¼ī(Na2CO3)ŌŚÉś²śÉś»īÖŠ¾ßÓŠ¹ć·ŗµÄÓĆĶ¾”£ŅŌĻĀŹĒŹµŃéŹŅÄ£ÄāÖĘ¼īŌĄķÖĘČ”Na2CO3µÄĮ÷³ĢĶ¼”£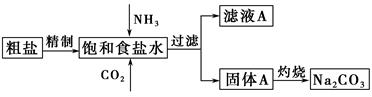
ŅŃÖŖ£ŗĻņ±„ŗĶŹ³ŃĪĖ®ÖŠĶØČėNH3”¢CO2ŗó·¢ÉśµÄ·“Ó¦ĪŖNaCl£«NH3£«CO2£«H2O=NaHCO3”ż£«NH4Cl”£Ēė»Ų“šŅŌĻĀĪŹĢā£ŗ
(1)“ÖŃĪÖŠŗ¬ÓŠµÄŌÓÖŹĄė×ÓÓŠCa2£«”¢Mg2£«”¢SO42£µČ”£
¾«ÖĘ³żŌӵIJ½ÖčĖ³ŠņŹĒa”ś ”ś ”ś ”śb(Ģī×ÖÄø±ąŗÅ)”£
a£®“ÖŃĪČܽā£¬ĀĖČ„³ĮŌü
b£®¼ÓČėŃĪĖįµ÷pH
c£®¼ÓČėBa(OH)2ČÜŅŗ
d£®¼ÓČėNa2CO3ČÜŅŗ
e£®¹żĀĖ
Ļņ±„ŗĶŹ³ŃĪĖ®ÖŠĻČĶØČėNH3£¬ŗóĶØČėCO2£¬ĄķÓÉŹĒ ”£
(2)×ĘÉÕ¹ĢĢåAÖĘNa2CO3ŌŚ (Ģī×ÖÄøŠņŗÅ)ÖŠ½ųŠŠ”£
a£®ŪįŪö b£®Õō·¢Ćó c£®ÉÕ± d£®×¶ŠĪĘæ
Ö¤Ć÷ĀĖŅŗAÖŠŗ¬ÓŠNH4£«µÄ·½·ØŹĒ ”£
¶ŌĀĖŅŗA½ųŠŠÖŲ½į¾§Äܹ»»ńµĆNH4HCO3£¬ĻņpH£½13ŗ¬Na£«”¢K£«µÄČÜŅŗÖŠ¼ÓČėÉŁĮæNH4HCO3Ź¹pH½µµĶ£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
(3)ĻĀĶ¼×°ÖĆÖŠ³£ÓĆÓŚŹµŃéŹŅÖʱøCO2µÄŹĒ (Ģī×ÖÄø±ąŗÅ)£»ÓĆb×°ÖĆÖʱøNH3£¬·ÖŅŗĀ©¶·ÖŠŹ¢·ÅµÄŹŌ¼ĮŹĒ (ĢīŹŌ¼ĮĆū³Ę)£¬ÉÕĘæÄŚæɼÓČėµÄ¹ĢĢåŹŌ¼ĮŹĒ (ĢīŹŌ¼ĮĆū³Ę)”£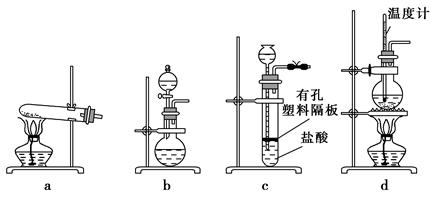
(4)Ņ»ÖÖĢģČ»¼ī¾§Ģå³É·ÖŹĒaNa2CO3”¤bNa2SO4”¤cH2O£¬Ä³Ķ¬Ń§ĄūÓĆĻĀĮŠĢį¹©µÄŹŌ¼Į£¬Éč¼ĘĮĖČēĻĀ¼ņµ„ŗĻĄķ²ā¶ØNa2CO3µÄÖŹĮæ·ÖŹżµÄŹµŃé·½°ø”£(ŅĒĘ÷×ŌŃ”)Ēė°ŃŹµŃé·½°øĢīČ«£ŗ
¹©Ń”ŌńµÄŹŌ¼Į£ŗ1.0 mol”¤L£1 H2SO4ČÜŅŗ”¢1.0 mol”¤L£1 BaCl2ČÜŅŗ”¢Ļ”°±Ė®”¢¼īŹÆ»Ņ”¢Ca(OH)2ČÜŅŗ”¢ÕōĮóĖ®
¢Ł³ĘČ”m1gĢģČ»¼ī¾§Ģåѳʷ£¬ČÜÓŚŹŹĮæÕōĮóĖ®ÖŠ”£
¢Ś ”£
¢Ū ”£
¢Ü¼ĘĖćĢģČ»¼ī¾§ĢåÖŠŗ¬Na2CO3µÄÖŹĮæ·ÖŹż”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¹¤ŅµÉĻŅŌ»ĘĶæó(Ö÷ŅŖ³É·ÖŹĒCuFeS2£¬ŌÓÖŹ²»ČÜÓŚĖ®ŗĶĖį)ĪŖŌĮĻ£¬ÖʱøĄ¶É«¾§ĢåG£¬Ęä»ÆѧŹ½ĪŖ[Cu(NH3)4]SO4”¤H2O£¬Éę¼°Į÷³ĢČēĻĀ£ŗ 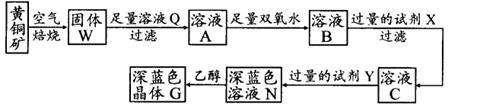
ŅŃÖŖ25”ꏱ£¬¼øÖÖ½šŹōĒāŃõ»ÆĪļµÄČܶȻż³£ŹżŗĶĶźČ«³ĮµķµÄpH·¶Ī§ČēĻĀ±ķ£ŗ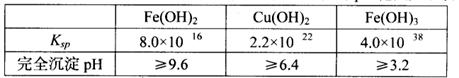
£Ø1£©»ĘĶæóŌŚæÕĘųÖŠ±ŗÉÕÄÜÉś³ÉĢśŗĶĶµÄµĶ¼ŪĮņ»ÆĪļ£¬Š“³öĘä·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ £»
£Ø2£©ŹŌ¼ĮXµÄ»ÆѧŹ½ĪŖ £»
£Ø3£©³£ĪĀĻĀ£¬0.1 mol£ÆLŹŌ¼ĮYµÄpH=11£¬ŌņøĆĪĀ¶ČĻĀ£¬ŹŌ¼ĮYµÄµēĄė³£ŹżĪŖ £¬ÓĆpHŹŌÖ½²āøĆČÜŅŗpHÖµµÄ·½·ØŹĒ £»
£Ø4£©ŌŚČÜŅŗNÖŠ¼ÓČėŅŅ“¼µÄÄæµÄŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻĀĆęŹĒÓŠ¹ŲĪļÖŹµÄ×Ŗ»Æ¹ŲĻµĶ¼(ÓŠŠ©ĪļÖŹŅŃŹ”ĀŌ)”£
ČōAĪŖµ„ÖŹ£¬EŌŚ³£ĪĀĻĀĪŖŅŗĢ壬CµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ78”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ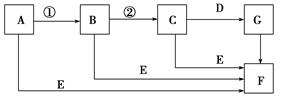
(1)»³öAµÄŌ×Ó½į¹¹Ź¾ŅāĶ¼________£¬FµÄµē×ÓŹ½ŹĒ________”£
(2)ĻĀĆę¶ŌCĪļÖŹ½į¹¹”¢ŠŌÖŹµÄĶʶĻÖŠ£¬²»ÕżČ·µÄŹĒ________”£
| A£®¾ĆÖĆÓŚæÕĘųÖŠ»į±ä³É°×É« |
| B£®¾ßÓŠĒæŃõ»ÆŠŌ |
| C£®¾§ĢåÖŠ“ęŌŚĄė×Ó¼üŗĶ¹²¼Ū¼ü |
| D£®ÓöŹŖČóµÄ×ĻÉ«ŹÆČļŹŌÖ½Ö»ÄÜŹ¹Ęä±äĄ¶É« |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻĀĶ¼±ķŹ¾µÄŹĒĻņNa2CO3ČÜŅŗÖŠµĪČėĻ”ŃĪĖįŹ±²śÉśCO2
µÄ¹ż³Ģ”£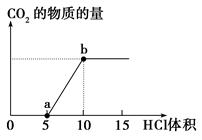
(1)Š“³öaµćŅŌĒ°·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ_____________________________________
(2)Š“³öaµ½bµć·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ_____________________________________
(3)ČōijNa2CO3ČÜŅŗÖŠŗ¬m mol Na2CO3£¬ĻņĘäÖŠµĪČėŅ»¶ØĮæµÄĻ”ŃĪĖį£¬Ē”ŗĆŹ¹ČÜŅŗÖŠCl£ŗĶHCOµÄĪļÖŹµÄĮæÅضČÖ®±ČĪŖ2”Ć1£¬ŌņµĪČėµÄĻ”ŃĪĖįÖŠµÄHClµÄĪļÖŹµÄĮæµČÓŚ________mol(ÓĆŗ¬×ÖÄømµÄ“śŹżŹ½±ķŹ¾)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ķ¼°Ęä»ÆŗĻĪļŌŚÉś²ś”¢Éś»īÖŠÓŠ¹ć·ŗµÄÓ¦ÓĆ”£
(1)ĶæɲÉÓĆČēĻĀ·½·ØÖʱø£ŗ
»š·ØĮ¶Ķ£ŗCu2S£«O2 2Cu£«SO2
2Cu£«SO2
ŹŖ·ØĮ¶Ķ£ŗCuSO4£«Fe=FeSO4£«Cu
ÉĻŹöĮ½ÖÖ·½·ØÖŠ£¬ĶŌŖĖŲ¾ł±»________(Ģī”°Ńõ»Æ”±»ņ”°»¹Ō”±)³ÉĶµ„ÖŹ”£
(2)Ó”Ė¢µēĀ·°åÉĻŹ¹ÓƵÄĶŠčŅŖ»ŲŹÕĄūÓĆ”£
·½·ØŅ»£ŗÓĆFeCl3ČÜŅŗ½žÅŻÓ”Ė¢µēĀ·°åÖʱøCuCl2”¤2H2O£¬ŹµŃéŹŅÄ£Äā»ŲŹÕ¹ż³ĢČēĻĀ£ŗ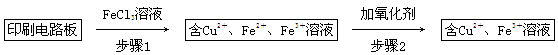

¢ŁÖ¤Ć÷²½Öč1Ėł¼ÓFeCl3ČÜŅŗ¹żĮæµÄ·½·ØŹĒ_________________________________”£
¢Ś²½Öč2ÖŠĖł¼ÓµÄŃõ»Æ¼Į×īŹŹŅĖµÄŹĒ______________________________________”£
A£®HNO3 B£®H2O2 C£®KMnO4
¢Ū²½Öč3µÄÄæµÄŹĒŹ¹ČÜŅŗµÄpHÉżøßµ½4.2£¬“ĖŹ±Fe3£«ĶźČ«³Įµķ£¬æÉŃ”ÓƵĔ°ŹŌ¼Į1”±ŹĒ________”£(Š“³öŅ»ÖÖ¼“æÉ)
¢ÜÕō·¢ÅØĖõCuCl2ČÜŅŗŹ±£¬ŅŖµĪ¼ÓÅØŃĪĖį£¬ÄæµÄŹĒ________(ÓĆ»Æѧ·½³ĢŹ½²¢½įŗĻ¼ņŅŖµÄĪÄ×ÖĖµĆ÷)£¬ŌŁ¾ĄäČ“”¢½į¾§”¢¹żĀĖ£¬µĆµ½CuCl2”¤2H2O”£
·½·Ø¶ž£ŗÓĆH2O2ŗĶĻ”ĮņĖį¹²Ķ¬½žÅŻÓ”Ė¢µēĀ·°åÖʱøĮņĖįĶŹ±£¬ĘäČČ»Æѧ·½³ĢŹ½ŹĒ£ŗ
Cu(s)£«H2O2(l)£«H2SO4(aq)=CuSO4(aq)£«2H2O(l)””¦¤H1£½£320 kJ”¤mol£1
ÓÖÖŖ£ŗ2H2O2(l)=2H2O(l)£«O2(g)¦¤H2£½£196 kJ”¤mol£1
H2(g)£«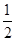 O2(g)=H2O(l)””¦¤H3£½£286 kJ”¤mol£1
O2(g)=H2O(l)””¦¤H3£½£286 kJ”¤mol£1
Ōņ·“Ó¦Cu(s)£«H2SO4(aq)=CuSO4(aq)£«H2(g)µÄ¦¤H£½________”£
(3)ÓūŹµĻÖ·“Ó¦Cu£«H2SO4=CuSO4£«H2”ü£¬ŌŚÄćČĻĪŖÄÜŹµĻÖøĆ×Ŗ»ÆµÄ×°ÖĆÖŠµÄĄØŗÅÄŚ£¬±ź³öµē¼«²ÄĮĻ(Ģī”°Cu”±»ņ”°C”±)”£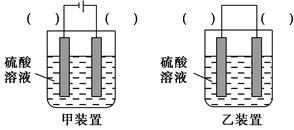
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com